Embryology, Week 1
- Article Author:
- Yusuf Khan
- Article Editor:
- Kristin Ackerman
- Updated:
- 4/30/2020 2:34:46 PM
- For CME on this topic:
- Embryology, Week 1 CME
- PubMed Link:
- Embryology, Week 1
Introduction
Human embryogenesis is a complicated process by which the fertilized egg develops into an embryo. During the first eight weeks of development, the concepts shifts from a single-celled zygote into a multi-leveled, multi-dimensional fetal body plan which utilizes primitively functioning organs. The continued growth and increased complexity within the organism during the first eight weeks are highly dependent upon cell signaling, proliferation, and differentiation. Because of the intricacies of human development, the processes divide into stages studied in a week-wise fashion. Week 1 marks the germinal stage of development, which refers to the timeline between fertilization and uterine implantation of a blastocyst. This article will explain the details taking place in the first week of human embryonic development.
Some of the important events that comprise the first week are:
- Gamete approximation
- Contact and fusion of gametes
- Fertilization
- Mitotic cleavage of the blastomeres
- Morula formation
- Blastocyst formation
- Implantation of the blastocyst
Development
Approximately two to five million spermatozoa are deposited into the vagina after sexual intercourse and must travel to their final destination within the fallopian tube for potential fertilization. The lifespan of sperm within the female reproductive tract ranges from three to seven days. Because sperm are terminally differentiated cells that lack transcription and translation machinery, they can not repair themselves upon damage in harsh environments. Sperm are subjected to physical stresses during ejaculation, contractions of the female tract, alteration in the pH environment, mucous, defenses of the female immune system, and must navigate a two-tract fallopian tube system. Thus, of the millions of sperm inseminated at coitus, only a few thousand reach the fallopian tubes to fertilize the secondary oocyte. In contrast, the female ovary releases one matured oocyte that has halted in metaphase of meiosis II into the fallopian fimbriae.
Gametes Approximation: Fertilization requires that sex cells are in close enough in proximation to bind to one another; thus, both sperm and egg must travel from their original deposition location to approximate at the fallopian tube. After spermatozoan deposition, the prostaglandin present in the semen along with oxytocin release during the coital reflex leads to uterine contraction. The mixture of sperms with the secretion of uterine glands is passively aspirated. It is worth remembering that the active motility of sperms does not help. Most sperm die within 24 hours of their release, as the spermatozoa are gradually reduced in number by barriers provided by abrupt constrictions at the cervix and uterine ostium. Of the hundreds of million sperm emitted at a single ejaculation, only 1% enter the uterus; a trip from the cervix to the oviduct takes from 30 minutes to 6 days. The oocyte travels via ciliary beats and rhythmical contractions of the musculature of uterine tubes. Finally, the sperms and ova reach to the ampulla of the fallopian tube.
Contact and Fusion of Gametes: Out of the thousand or fewer sperms reaching the ovum, only one fully penetrates the oocyte. The remaining sperms are engaged in the disintegration of the three barriers around secondary oocyte by an enzyme – hyaluronidase, which is present in the acrosomal cap of sperm. The three barriers around the secondary oocyte are as follows: corona radiata, zona pellucida, and the vitelline membrane. Nevertheless, before the actual fertilization (penetration) takes place, the spermatozoa have to undergo a process called capacitation, followed by an acrosomal reaction (discussed in the Cellular Section). These two processes help the sperm to acquire the capability to penetrate the ovum.[1]
The cytoplasm of mature ovum contains two pronuclei – sperm head with its nuclear envelope form male-pronucleus and nucleus of mature ovum forms female-pronucleus. Both contain 1N haploid chromosomes. The DNA replicates from 1N to 2N DNA duplex. The pronuclear envelopes disappear, and the chromosomes of each arrange themselves in the equatorial plane.
Fertilization: Fertilization is a process of fusion of a mature male and female gametes to form a single cell – the zygote. A spermatozoon is a male gamete, and the ovum is a female gamete. Both the male and female gametes are haploid containing 23 chromosomes.[2] However, the zygote becomes diploid with the fusion of both the gametes, thus restoring the number of chromosomes to 23 pairs (46 chromosomes). Fertilization usually occurs in the ampullary part of the fallopian (uterine) tube.
Mitotic Cleavage of the Blastomeres: After fertilization, the resulting one-celled zygote will rapidly undergo multiple mitotic cleaves as it travels four days toward the uterus. One should recall that the zygote is much larger compared to many cells of the body. Thus the process of cleavage or segmentation results in the production of blastomeres, which restores the standard size of cells by a progressive reduction of cytoplasmic volume of the zygote. Once the cleavage begins, we can expect the following changes over time: two-cells stage (approximately one day), four-cells stage (approximately two days), twelve-cells stage (approximately three days), and finally sixteen-cells stage (approximately four days).
Morula Formation: Morula formation occurs four days after fertilization and first appears as a 16 to 32 celled mass still surrounded by the zona pellucida. By this stage, the cells will be compacted (see cellular events below) to a solid ball of cells that are similar in size and structure. Medically, this is often known as the final stage before the formation of a fluid-filled cavity called the blastocoel cavity, which precedes blastula formation. Recent time-lapse microscopy observations suggest that compaction may represent an important checkpoint for human embryo viability, through which chromosomally abnormal blastomeres are sensed and eliminated by the embryo. Compaction is critical because it sets anatomical differences between cells (inner vs. outer), which ultimately determines their fate. The group of cells present in the center of the morula will eventually give rise to the development of the embryo proper.[3] The cells which are at the periphery are outer cell mass cells and are critical in the cavitation of the morula to transition to a blastocyst.
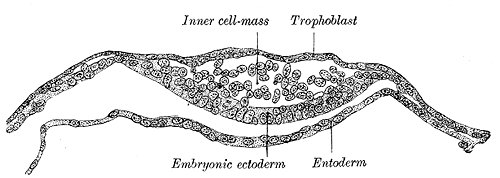
Blastocyst Formation: By the blastocyst stage (day five after fertilization), the embryo has reached 50 to 150 cells and is starting to strain at the confines of the zona pellucida. This straining is not merely due to cell division but also active pumping of fluid by outer cell mass cells into the inner space of the blastocyst, which forms a cavity or blastocoel. The filling of this space with fluid expands the blastocyst; thus, the term expanded blastocyst. Before the creation of this fluid space, the embryo is called non-expanded. Expansion functions to thin the zona pellucida, and eventually ruptures the pellucida to let the blastocyst escape or hatch from the zona pellucida. Blastocyst expansion also functions to allow a large amount of fluid entering the space to shift a grouping of cells off to one side of the hollowed interior. This cellular mass is a grouping of embryonic stem cells with unrestricted developmental potential termed the inner cell mass (ICM) within the blastocyst. The ICM has the cells that will give rise to the actual fetal cells. The other cells surrounding and protecting the ICM and that line the inner side of the zona pellucida are the trophectoderm cells, which give rise to the fetal part of the placenta. Thus, hallmarks of successful blastocyst formation are the fluid-filled blastocoele, the ICM, and the trophectoderm cells which have fully differentiated into trophoblast.
Apposition of the Blastocyst: Implantation is the process of the blastocyst embedding into the endometrial lining of the uterus, which occurs completely in Week 2 of Development. Implantation requires the complete removal of the zona pellucida from the blastocyst once the conceptus has entered the uterine cavity. Early pellucida disappearance can lead to tubal pregnancies. Usually, the human blastocyst implants in the endometrium near the fundus along the anterior or posterior wall of the uterus in between the openings of the glands.[4][5] If implantation is too low, it can lead to placenta previa.[6] As we know, there are three distinct layers within the wall of the endometrium: stratum compactum, stratum spongiosum, and stratum basale. At the time of implantation, the uterus has been primed for implantation through decidualization. The uterus is in the secretory phase, which is defined by high levels of estrogen and progesterone that function to coil glands and arteries, allowing the tissue to become succulent.[7][7] Early implantation initiates by the apposition or the approach of the blastocyst to the uterine wall via cell adhesion factors and polarity signaling. The blastocyst orients with the ICM to enter the uterine lining first. Immediately prior to implantation, the outer trophoblast cells begin to differentiate to allow full implantation to proceed into Week 2 of development.
Cellular
Gamete Approximation: Gamete approximation occurs via anatomical movements of the oocyte and sperm toward the ampulla of the fallopian tube. Additionally, approximation requires chemotactic events where the oocyte and surrounding cumulus cells release chemical attractants into the local environment to which only capacitated sperm can respond. The secondary oocyte itself and the cumulus cells release progesterone and other nonpeptide signaling molecules. As mentioned, only spermatozoa that have undergone capacitation events are guided by chemoattractants released by the ovum environment. Capacitation is a set of physiological and biochemical changes (see details in the Biochemical Section) that initiate after moving through the cervical mucus and finalizes in the fallopian tube. Capacitation renders the sperm capable of fertilizing an oocyte by modifying the head and tail of the sperm. After capacitation and before the approach to the oocyte, the sperm must also undergo an acrosomal reaction. The acrosomal reaction only occurs in capacitated sperm and is a set of changes to the acrosome, which is a Golgi-derived organelle located between the cell membrane and nuclear envelope. Generally, the acrosomal vesicle fuses with the plasma membrane to exocytose substances, mainly enzymes that facilitate fertilization by degrading the oocyte's corona radiata, zona pellucida, and the vitelline membrane.
Oocyte contact and fusion of gametes: The cumulus oophorus is a layer of loosely packed follicle cells connected by hyaluronic acid. As the sperm approaches the oocyte, the cells of cumulus oophorus and corona radiata surrounding the oocyte are moved by the liberation of hyaluronidase from the acrosomal cap of the sperm to enzymatically dissolve the hyaluronic acid and expose the zona pellucida. As a second barrier, the zona pellucida is a thick striated membrane made up of three glycoproteins – ZP1, ZP2, and ZP3 that are synthesized by the oocyte.[8] The sperm head binds to ZP3 and ZP2 receptors and induces acrosome reaction to release acrosin enzyme, which digests zona pellucida. Now the sperm head fuses with a vitelline membrane, which acts as the third barrier. The two disintegrin peptides of sperm head open a gateway for a single sperm to enter the oocyte cytoplasm.
Upon the entrance of the single sperm, a series of cellular events termed cortical reaction transpires to generate an impermeable zona pellucida, which prevents polyspermy. Cortical granules are released and secrete serine proteases, peroxidases, and glycosaminoglycans to cut proteins connections to remove receptors, harden the vitelline envelop, and attract water into the perivitelline space which generates a gap to form the hyaline layer.
A key signaling event resulting from the binding of a sperm to its ZP receptor in the zona pellucida is an increase in the level of cytosolic Ca within the egg. Increase calcium is needed to direct the cortical reaction and also to complete fertilization.
Fertilization: The process of fertilization gives rise to five critical cellular events:
- There is the completion of meiosis II within the female gamete directed by calcium-induced activation of the anaphase-promoting complex.
- There will be a restoration of a diploid number of chromosomes in the zygote.
- The determination of chromosomal sex is now complete.
- The sperm entry point may localized polarity to the determination of early axis formation.[9]
- Finally, there is an initiation of the cleavage division of the zygote.
Mitotic Cleavage of the blastomeres: Early cleavage events are synchronous and driven by maternal RNA and protein instruction until the eight-celled stage is complete. Upon the completion of eight blastomeres, the cells show increased adherence via tight junctions and are totipotent with functional apparatus which displays cell autonomy. The rapid rate of blastomeric division initiates via changes in the cell cycle, specifically the upregulation of cyclins. In IFV studies, the length and timing of the cell cycle have been shown to be one of the most important determinants in survivability.
Morula Formation: Dynamic cellular processes, such as filopodia formation and cytoskeleton-mediated cell-to-cell interactions, intervene to allow cell compaction and formation of the blastocoel. Compaction is driven by E-cadherins and Ca2+-driven signal transduction to instruct cell boundaries to disappear. Cell boundaries then progressively disappear until the embryo is fully compacted. Finally, at the late compaction stage, the cell boundaries reappear, the number of blastomeres increases, and the cavitation begins. At the same time that cell boundaries are reappearing, the differential orientation of cleavage planes, cell polarity, and physical forces interact and cooperate to position blastomeres either internally or externally, thereby influencing their cellular fate. Cells of the outer cell mass, upregulate Na+ transporters actively pumping Na+ into the interior space of the morula. Via osmosis, water actively follows the increased Na+ gradient, thus creating a sizeable fluid-filled cavity called blastocoele.
Blastocyst Formation: One of the first key developmental patterning decisions in the morula to blastocyst transition is to determine what becomes inner cell mass vs. trophectoderm. Studies in mice indicate that the upregulation of the transcription factors such as Oct 4, FGF, Nanog define pluripotent stem cells of the ICM, with trophectoderm cells upregulating Cdx-2 Eomes and Tead 4.
Apposition of the Blastocyst: Literature indicates that chemokines are involved in the apposition of the blastocyst to the correct location within the uterine lining for implantation. The interleukin family of signaling factors, along with leptin, IGF, and DKK-1, have all been implicated in early implantation events.
Biochemical
Capacitation: The process of fertilization is preceded by significant biochemical and physiological changes in the sperm, termed as capacitation. The reactions occur in the female reproductive tract, which further facilitates the acrosome reaction. At the physiological level, capacitation involves two signaling events, namely fast and slow. The fast events are the vigorous and incoherent movement of the sperm flagella, while the slow events are the discrete modulations in the sperm mobility patterns, termed as hyperactivation. Another essential feature of capacitation is signaling through the protein tyrosine phosphorylation. Capacitation initiates by a series of signaling events involving the phosphorylation of phosphatidyl-inositol-3-kinase (PI3K), through protein kinase A (PKA) dependent cascades leading to its activation. PI3K is downregulated by protein kinase C-α (PKCα).
PI3K inactivation is necessary at the beginning of capacitation. However, as capacitation proceeds, PI3K is activated by the degradation of PKCα as well as PP1γ2. The activation of PKCα depends on the concentration of cyclic adenosine monophosphate (cAMP) produced by the bicarbonate-dependent soluble adenylyl cyclase. Another significant biochemical change is the removal of cholesterol from the plasma membrane of the sperm head as a feature of slow events of capacitation. This removal increases the permeability of the sperm towards calcium and bicarbonate ions, which hyperpolarizes the plasma membrane. Hyperpolarization is central to the modulation of protein phosphorylation and protein kinase activities. This activation of PKA causes PI3K activation and hence leads to an increase in actin polymerization. PI3K activation is essential for the development of hyperactivated motility of sperm necessary for successful fertilization. PKCα is active at the beginning of capacitation, resulting in PI3K inactivation. During capacitation, PKCα, as well as PP1γ2, is degraded by a PKA-dependent mechanism, allowing the activation of PI3K.[10]
Acrosome reaction: The second prerequisite essential to fertilization of sperm with oocyte is the acrosome reaction. The membrane surrounding the acrosome dissolves to fuse with the plasma membrane of the sperm's head; thus, the contents of acrosome are released. These contents consist of surface antigens necessary for binding to the cell membrane of the egg, and lytic enzymes for breaking inside the egg's tough layers for the fertilization to occur. The process is facilitated by the influx of calcium and is entirely dependent on increased intracellular calcium.
The acrosome reaction is an exocytotic mechanism that releases soluble lysins of the acrosome. This release helps in developing a path in the egg coat, allowing penetration. The lysins are similar to the enzymatic content that includes acrosin, acrogranin, hyaluronidase, usually present as a part lysosome and peroxisomes.[11] Acrosin is the major protease present in a mature sperm acrosome. It is stored in its precursor form, proacrosin and is released onto the zona pellucida upon stimulation. The zymogen, proacrosin, is then processed into its active form, β-acrosin. This causes the lysis of the zona pellucida, thus facilitating the entry of the sperm through the innermost glycoprotein layers of the ovum.[12] Protein C inhibitors are instrumental in the regulation of acrosin. These inhibitors are present in the male reproductive tract at higher concentrations than in the blood plasma, and function to inhibit the proteolytic activity of acrosin. This provides a protective role in the case of premature release or degeneration of the spermatozoa within the male reproductive tract.[13]
Many ligands, especially hormones from the female reproductive tract, are demonstrated to modulate the acrosome reaction through receptor binding. These compounds can be either activating or inhibitory to the acrosome reaction. Progesterone, catecholamines, insulin, leptin, prolactin, angiotensin are among the inducers, while oestradiol and epidermal growth factor are capable of inhibiting acrosome reaction. Interestingly, gamma-aminobutyric acid, an inhibitor of the central nervous system, acts as an activator to the acrosome reaction. The female hormone progesterone acts by inhibiting the acrosome reaction, possibly due to the increase in cytoplasmic protein phosphorylation through the modulation of intracellular calcium concentration. However, a decrease in plasma membrane cholesterol probably determines the extent of the response of progesterone to human sperm.[14]
Clinical Significance
Hydatidiform mole is a type of gestational trophoblastic disease resulting from abnormal fertilization of an oocyte where the sperm fertilizes an egg lacking the female pronucleus, leading to trophoblastic proliferation without any proliferation of the embryoblast or embryo proper. The cells of trophoblast continue to function normally by secreting human chorionic gonadotropin (hCG), which will trigger a positive pregnancy test. Hydatidiform mole is often attributed to maternal gene defects and is more common in women 36 to 44 years of age with a history of gestational viability issues. The clinical presentation will be very vague, including uterine enlargement more than the gestational age, vaginal spotting, severe nausea, and vomiting. Hydatidiform mole is detected via ultrasound examination and presents with normal placental development, a mass of tissue with no apparent fetal features, and the absence of heart sounds.[15][16]
Ectopic pregnancies [17] is defined as implantation outside of the uterus. The leading cause of ectopic pregnancies is the early disappearance of the zona pellucida allowing for opposition and attachment to occur in a variety of anatomical locations. Other causes include pelvic inflammatory diseases (PID), scarring, and tubal defects, etc. Ectopic pregnancies classify into many types depending on the site of implantation:
- Ampullary region of the fallopian tube: Typically, fertilization takes place in the most dilated part of the uterine tube, the ampulla. Once fertilization takes, the zygote will generally travel to the uterine cavity and implant in the wall of the uterus near the fundus. In some cases, the zygote does not travel to the uterine cavity but stays at the site of fertilization, fixes to the wall of the ampulla, and starts growing within it. Attachment to the fallopian tube is the most common abnormal site of implantation, approximating almost 70% to 80%.[18]
- Tubal implantation: In this condition, the implantation of the zygote takes place in any other part of the uterine tube, other than the fertilization site. These are the second most common abnormal implantation site, approximating 12% of ectopic pregnancies.[19]
- Within the abdominal cavity: In certain rare conditions, the zygote moves in the opposite direction exiting the fallopian tube into the abdominal cavity (pouch of Douglas, spleen, omental, retroperitoneal, and liver); this occurs in approximately 1.3% of all abnormal implantation.[20]
- Interstitial implantation: 4% of ectopic pregnancies present in the narrowest part of the uterine tube and present with excessive hemorrhage. Thus, mortality and morbidity are 7% higher in interstitial implantation than all other types of ectopic pregnancies.[20]
- Cervical pregnancy (Internal os): In almost 0.2% of cases ectopic pregnancy, the zygote travels too far into the internal os of the cervix, implants and begins to grow. Embryonic growth induces pain as the cervix has minimal capacity to dilate.[21]
- Ovarian implantation (0.2%): The zygote travels in the opposite direction and gets implanted in the ovary in almost 0.2% of cases.[22]
Generally, ectopic pregnancies are identified very early but can be life-threatening if detected too late. Treatment includes surgical intervention to remove the conceptis.
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
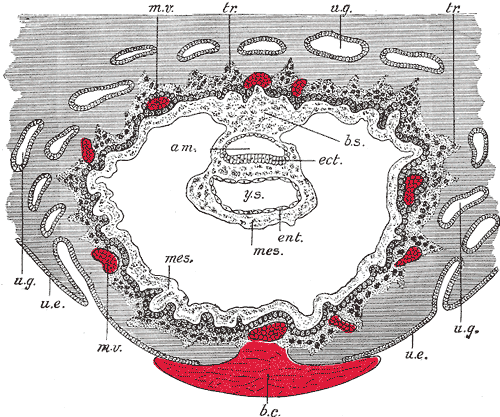
Development of the Fetal Membranes and Placenta, Section through ovum imbedded in the uterine decidua. Semi Diagrammatic, Amniotic Cavity, Blood clot, Body stalk, Embryonic Ectoderm, Entoderm, Mesoderm, Maternal vessels, Trophoblast, Uterine epithelium, Uterine Glands, Yolk sac
Contributed by Gray's Anatomy Plates