Neuroanatomy, Edinger–Westphal Nucleus (Accessory Oculomotor Nucleus)
- Article Author:
- Madeline Heiland Hogan
- Article Author:
- Suriya Subramanian
- Article Editor:
- Joe M Das
- Updated:
- 9/19/2020 7:19:54 PM
- For CME on this topic:
- Neuroanatomy, Edinger–Westphal Nucleus (Accessory Oculomotor Nucleus) CME
- PubMed Link:
- Neuroanatomy, Edinger–Westphal Nucleus (Accessory Oculomotor Nucleus)
Introduction
The Edinger-Westphal (EW) nucleus, which is part of the oculomotor nuclear complex (ONC), was first described in the literature in the 17th century.[1] Recently, researchers have discovered two different cell populations within the EW nucleus.[2] The EW nucleus subdivides into the EW preganglionic (EWpg) population and the EW nucleus centrally projecting (EWcp) population. However, accepted nomenclature for these two groups varies.[1][2]
The EWpg is what is thought of as the classic ONC—sending parasympathetic nerve fibers towards the eye. It is located in the midbrain immediately dorsal to the oculomotor nucleus near the level of the superior colliculus, which is why it is often included in the overarching term oculomotor complex.[3] The EWcp population of cells (the non-preganglionic EW cells), also referred to as the subgriseal paramedian midbrain neuronal stream to reflect their actual path, differ in function from the EWpg nucleus.[4][5] However, the name subgriseal paramedian midbrain neuronal stream has not found favor in the literature due to the complexity of the name and belief that the EW nucleus consisted of only one cell type. These two bodies of cells, while both referred to as the EW nucleus and intermingled from a structural standpoint in the midbrain, have fundamentally different roles in function.
Structure and Function
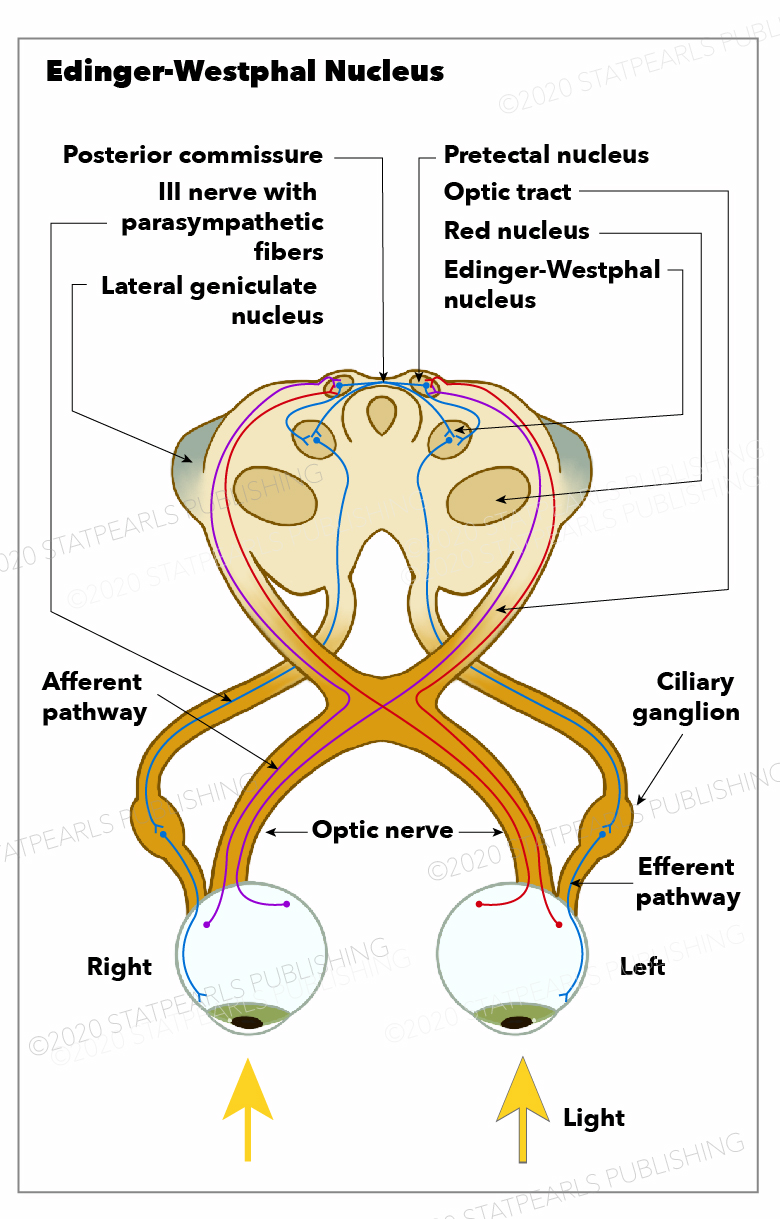
The EWpg nucleus houses choline acetyltransferase positive cell bodies responsible for the parasympathetic innervation of the eye.[6] The EWpg nucleus is comprised of preganglionic cell bodies that track along the course of the oculomotor nerve (cranial nerve III) towards the postganglionic ciliary bodies.[6] These cell bodies participate in the light reflex pathway that leads to pupillary constriction when the retina has exposure to light.[6][7] The EWpg nucleus receives input from the locus ceruleus in response to the light hitting the retina, which then prompts the nucleus to send a forward signal which synapses at the postganglionic cells of the ciliary ganglion (CG).[6] This synapse between the EWpg nerve fibers and the ciliary ganglion postganglionic cell bodies is a nicotinic synapse with acetylcholine as the neurotransmitter.[6]
In response to the signal from the EWpg, the postganglionic ciliary bodies relay the signal along their axons by way of the ciliary nerves towards the eye. This relay leads to the innervation of the sphincter pupillae (causing miosis) and ciliary muscles (ocular accommodation). The constriction of the pupil moderates the amount of light the retina is exposed to, which is the efferent limb of the pupillary light reflex. Additionally, the contraction of the ciliary muscles leads to the relaxation of the zonular fibers, allowing for increased convexity of the lens and, subsequently, an increase in refractive power and accommodation.[6]
Since the discovery of the two cell populations of the EW nucleus, the function of the EWcp has been a subject of ongoing research. The EWcp is located medial and dorsal to the OCN in the midbrain and is comprised of a collection of peptidergic neuron cell bodies.[6][8][2] It is known that a large population of these cells are urocortinergic neurons, which are positive for the neuropeptide urocortin-1 (part of the corticotropin-releasing factor family) and negative for choline acetyltransferase.[4] These differ in immunohistochemistry from the cholinergic parasympathetic neurons of the EWpg, which are choline acetyltransferase positive. The EWcp nucleus is a significant contributor to the amount of urocortin-1 neuropeptide in the brain.[9] Studies have shown that this nucleus is involved in stress adaptation, anxiety, and pain.[10] However, the thought is that the response to stress by the EWcp nucleus is separate from that of the hypothalamic-pituitary-adrenal (HPA) axis.[11]
Embryology
Nucleogenesis in the brain during embryologic development is a subject of ongoing research. The thought is that brain nuclei develop as part of neuromeres and arcs (distinct columns of cells in the brain with high levels of acetylcholinesterase).[12] As part of the midbrain, the EW nucleus develops with the midbrain arc, which is under the control of the homeobox genes Sonic Hedgehog and FGF8.[12] Post-mortem studies in neonates who suffered prenatal hypoxia have shown there to be an expression of urocortin-1 from the EWcp as early as 34 weeks gestation—demonstrating both the development of the EW nucleus at this age and specifically the EWcp role in stress response.[8]
Blood Supply and Lymphatics
While there is not a single blood supply to the EW nucleus, it receives its blood supply via vessels that feed the midbrain and brainstem. These blood vessels are part of the vertebrobasilar circulation and include the basilar artery, superior cerebellar artery, and posterior cerebral artery.[13] The lymphatic drainage of the brain consists of the perivascular pathway (basement-membrane drainage system), glymphatic pathway, and cerebrospinal fluid drainage via meningeal lymphatic vessels and cervical lymph drainage routes.[14]
Nerves
The EWpg nucleus projects parasympathetic preganglionic neurons, which synapse on the postganglionic ciliary bodies.[6] [7] The axons of the postganglionic ciliary bodies are the short ciliary nerves that innervate the sphincter pupillae and the ciliary muscles of the eye.[6] The EWcp nucleus sends projections to the interpeduncular nucleus (a component of the limbic midbrain), lateral hypothalamus, lateral septum, raphe, and preganglionic sympathetic neurons in the spinal cord; however, these projections are not explicitly named nerves.[5] While the oculomotor nucleus is a separate complex, the preganglionic projections to and postganglionic projections from the ciliary ganglion do course along CNII.[6] The oculomotor nucleus houses the motor neurons that innervate the superior, inferior, and medial recti as well as inferior oblique and levator palpebrae ocular muscles.[3]
Muscles
As discussed above, the short ciliary nerves innervate the sphincter pupillae (miosis) and the ciliary muscles (lens accommodation secondary to zonular fiber relaxation).[6]
Physiologic Variants
Much of the information we know today about the EW nucleus was originally studied in monkeys, mice, and birds. For instance, cellular morphology in pigeons differs from mice and human morphology, which have similar patterns of distribution of urocortin-1 positive neurons of the EWcp.[4] However, there remains more opportunity for the variations in cell populations in the EW nucleus to be studied in humans.
Surgical Considerations
Brainstem and midbrain surgery is technically challenging.[15] Surgical considerations for the EW nucleus consist of any surgical intervention of the brainstem or midbrain structures.
Clinical Significance
One clinical implication of the EW nucleus is its association with Alzheimer's Disease (AD). Being that there are more than 5.4 million Americans estimated to be affected by AD and that this neurodegenerative disease is one of the leading causes of death in the United States, this relationship could prove to be an important finding in the understanding of the disease.[16] Degenerative changes in the early course of the disease are known to affect the hippocampus and entorhinal cortex, but another early target is the neurons of the EW nucleus.[17][16] The density of the dendritic spines of the EW neurons was significantly reduced in the post mortem analysis of AD-affected brains when compared to unaffected age-matched controls.[17] This finding clinically correlates with exaggerated pupillary responses to cholinergic antagonists seen early on in the course of the disease in individuals with AD, and being that the EW nucleus is an early target of degeneration, this response could be a useful tool in the early clinical diagnosis of AD.[17]
Other clinical considerations would include the presence of structural lesions that could lead to the compression of the nucleus. These lesions include tumors, aneurysms, and impaired CSF outflow leading to cerebral aqueduct enlargement.
Other Issues
Many of the issues regarding the understanding of the EW nucleus involve the discovery of the EWcp, which changed the classic definition of the EW nucleus, as it was solely associated with parasympathetic control of the eye for decades. With time and more research, the terminology of the EWpg and EWcp will gain recognition and acceptance throughout the scientific community.