Neuroanatomy, Semilunar Ganglion
- Article Author:
- Megan Yu
- Article Editor:
- Charles Donohoe
- Updated:
- 7/31/2020 3:17:27 PM
- For CME on this topic:
- Neuroanatomy, Semilunar Ganglion CME
- PubMed Link:
- Neuroanatomy, Semilunar Ganglion
Introduction
The semilunar sensory ganglion (also known as the trigeminal ganglion or Gasserian ganglion) is a thin, crescent-shaped structure situated in Meckel’s cave within the middle cranial fossa.[1] The semilunar ganglion plays a critical role in expressing various neuropeptides and signaling molecules that are important in gene expression, modulation of sensory information, and peripheral and central sensitization.[2]The ganglion contains the cell bodies of the sensory roots of the three major divisions of the trigeminal nerve (CN V); the ophthalmic (V1 sensory), maxillary (V2 sensory) and mandibular (V3 motor and sensory) branches of the motor root originates from cells located in the masticator motor nucleus of the trigeminal nerve located in the mid pontine portion of the brainstem. The trigeminal nerve is the largest of the 12 cranial nerves.
Structure and Function
Located at the anterior, inferior, and lateral borders of Meckel’s cave, the semilunar ganglion is a crescent-shaped structure that is essential for releasing many neuropeptides and signaling molecules. As a potent vasodilator, calcitonin gene-related peptide is the most prevalent among these neuropeptides and plays a vital role in the pathophysiology of cluster and migraine headaches.[3] The semilunar ganglion also expresses many signaling molecules, including adenosine triphosphate, neurotrophic factors, nitric oxide, and cytokines, that target nearby neurons or satellite glial cells. These signaling molecules and neurotrophic factors play an important role in peripheral and central sensitization, such as the case with primary headaches.[2]
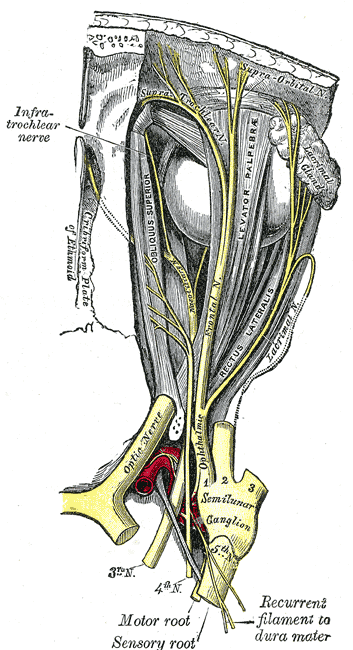
The convex border of the semilunar ganglion is located at the anteroinferolateral walls of Meckel’s cave, while the concave border, which is also known as the sinus ganglion, is located posterosuperomedially in the direction of the trigeminal cistern and the ostium of the Meckel’s cavity. A layer of dura propria and arachnoid that surrounds the semilunar ganglion. The posteromedial portion of the semilunar ganglion is situated in the trigeminal cistern while the anteroinferior portion is located outside of the trigeminal cistern as it adheres closely to the dura of the Meckel’s cavity medially and the dura of the temporal fossa laterally.[1] The semilunar ganglion can segregate into three divisions: the ophthalmic (V1), which is the dorsal division, maxillary (V2), which is the intermediate portion, and mandibular (V3), which is the ventral division. The ophthalmic portion exits the semilunar ganglion and enters the orbit via the superior orbital fissure. It separates into the supraorbital, supratrochlear, and nasociliary nerves that provide innervation to the nose and forehead. The maxillary portion leaves the middle cranial fossa and passes through the orbit via the inferior orbital fissure, and includes multiple branches, such as the infraorbital, palatine, zygomatic, and superior alveolar nerves. The mandibular division separates into the buccal, auriculotemporal, lingual, and inferior alveolar nerves, and is the only division that provides sensory and motor components.[4]
Embryology
The semilunar ganglion contains neurons that arise from two trigeminal placodes and the neural crest. In the chick, they are known as the ophthalmic and maxillomandibular trigeminal placodes, whereas in Xenopus, they are known as the profundal and trigeminal placodes. In the chick, the ophthalmic placode has a small area of ectodermal thickenings divided across a broad area rostral to rhombomere one and gives rise to postmitotic neurons.[5] The maxillomandibular placode is a much thicker area of ectodermal thickenings that is ventral to rhombomeres two and three and give rise to mitotically active neurons.[6] The first condensation of the semilunar ganglion forms by neural crest cells. In the chick, the ophthalmic placode can express Pax3 (also known as the paired domain transcription factor gene), Fgfr4, (also known as the fibroblast growth factor receptor 4 gene), and Neurogenin2 (also known as the proneural transcription factor gene) while the maxillomandibular trigeminal placode expresses Ngn1 (also known as the proneural transcription factor gene). Neurogenesis in the ophthalmic placode starts in the ten-somite stage with the expression of Ngn2. Researchers also found that even though the degree of separation between the ophthalmic and maxillomandibular trigeminal placodes augments with time, a definitive border between them does not exist even at the 16-somite level. Moreover, some cells along the border between the two placodes express Pax3 and Ngn1, which suggest these cells respond to ophthalmic and maxillomandibular placode-inducing signals.[6]
Blood Supply and Lymphatics
Blood supply to the semilunar ganglion comes from the middle meningeal artery, accessory middle meningeal artery, the inferolateral trunk of the internal carotid artery, and the tentorial branch of the superior cerebellar artery. The trigeminal nerve root acquires vascular supply from the superolateral pontine artery, anterior inferior cerebellar artery, inferolateral or posterolateral pontine arteries, superior cerebellar artery, basilar artery, and trigeminocerebellar artery. The motor root and the ophthalmic portion of the sensory root usually receive blood supply from the superolateral pontine artery while the maxillary portion receives blood supply from the superolateral and inferolateral pontine arteries and the mandibular portion of the anterior inferior cerebellar artery. Most of the blood supply to the semilunar ganglion is supplied by the middle meningeal artery and inferolateral trunk of the internal carotid artery.[7]
Muscles
Only the mandibular branch of the trigeminal nerve has a motor component. The mandibular branch provides motor innervation to the facial muscles that are involved in mastication, including the master, temporalis muscle, and the lateral and medial pterygoids. It also separates into branches that innervate the tensor tympani, tensor vela palatine, mylohyoid, and the anterior part of the digastric muscle.[4]
Physiologic Variants
There are reports indicating that the size of the semilunar ganglion varies from 14 to 22mm in length and 4 to 5 mm in thickness. However, after taking into account the concave structure of the semilunar ganglion, the semilunar ganglion is 1.5 to 2 mm in thickness.[1]
Surgical Considerations
Nerve block utilizing anatomic landmarks or image-guided by fluoroscopy or computed tomography of the semilunar ganglion may be effective for patients who have refractory facial pain due to trigeminal neuralgia, certain headaches, conditions such as recalcitrant herpes zoster ophthalmicus and postherpetic neuralgia who have not responded well to medical management were generally carbamazepine as the drug of choice. The procedure may also be an option in cases where neurosurgical management is not practical.[4]
Clinical Significance
As stated previously, the semilunar ganglion plays a critical role in expressing various neuropeptides and signaling molecules that are important in gene expression, modulation of sensory information, and peripheral and central sensitization. As a potent vasodilator, calcitonin gene-related peptide is the most prevalent among these neuropeptides and plays an essential role in the pathophysiology of cluster headaches. The semilunar ganglion also expresses many signaling molecules, including adenosine triphosphate, neurotrophic factors, nitric oxide, and cytokines, that target nearby neurons or satellite glial cells.[3]
The semilunar ganglion is also known to become latently infected with the herpes simplex virus type-1 (HSV-1).[8] HSV-1 would periodically reactivate, whereas the varicella-zoster virus rarely reactivates itself. The reactivation of this virus generally results in various cranial nerve disorders, including vestibular neuritis, Bell’s palsy, and herpes labialis. Also, as stated previously, neural of the semilunar ganglion may be effective for patients who have refractory facial pain, certain headaches, and certain conditions, such as recalcitrant herpes zoster ophthalmic and postherpetic neuralgia who have not responded well by other means.[4] The semilunar ganglion also has involvement in the often underappreciated perineural spread of tumors, which are the most common malignant in which there is delayed diagnosis. This situation is a well-recognized phenomenon in head and neck cancers, including squamous cell carcinoma, adenocystic carcinoma, lymphoma, and rhabdomyosarcoma.
The semilunar ganglion is also known to incur damage in patients with trigeminal trophic syndrome (TTS).[9] Commonly seen in elderly patients, TTS is a form of chronic facial ulceration that usually appears in a unilateral crescent-shaped form near the nasal ala and, in more serious cases, in the jaw, forehead, cheeks, and lips. Patients with TTS may report paresthesias, including burning or shooting pains. The general belief is the ulceration might be the result of the patient touching their face consistently, especially during sleep. Some treatment options include altering the patient’s behavior, occlusive dressings, oral psychotropic medications, and arm splints.[9]