Physiology, Cerebral Spinal Fluid
- Article Author:
- Lauren Telano
- Article Editor:
- Stephen Baker
- Updated:
- 7/14/2020 8:29:20 PM
- For CME on this topic:
- Physiology, Cerebral Spinal Fluid CME
- PubMed Link:
- Physiology, Cerebral Spinal Fluid
Introduction
Cerebrospinal fluid (CSF) is an ultrafiltrate of plasma contained within the ventricles of the brain and the subarachnoid spaces of the cranium and spine.[1] It performs vital functions, including providing nourishment, waste removal, and protection to the brain.[2] Adult CSF volume is estimated to be 150 ml, with a distribution of 125 ml within the subarachnoid spaces and 25 ml within the ventricles. CSF is predominantly secreted by the choroid plexus, with other sources playing a more poorly defined role. Its secretion varies between individuals with adult production, usually ranging between 400 to 600 ml per day. The constant secretion of CSF contributes to complete CSF renewal four to five times per 24-hour period in the average young adult. The reduction of CSF turnover may contribute to the accumulation of metabolites seen in aging and neurodegenerative diseases. The composition of CSF is strictly regulated, and any variation can be useful for diagnostic purposes.[1]
Cellular
Seventy to eighty percent of CSF production is via a network of modified ependymal cells known as the choroid plexus (CP).[1] The CP is a highly specialized simple cuboidal epithelium continuous with ependymal cells lining the ventricles of the brain. This simple cuboidal epithelium surrounds clusters of fenestrated capillaries allowing for the filtration of plasma.[3] Cells of the CP have dense microvilli present on their apical surface. They are interconnected via tight junctions, creating a blood-CSF barrier that helps to control the composition of CSF.[1]
Because there is no appreciable barrier between the CSF and the extracellular space of the brain (ECSB), the blood-CSF barrier also serves to regulate the environment of the brain.[2] Larger substances such as cells, protein, and glucose are not allowed passage, whereas ions and small molecules such as vitamins and nutrients can pass into the CSF relatively easily.[4] Water is allowed passage through the CP epithelium via epithelial AQP1 channels. Substances that may not pass through the blood-CSF barrier but are needed by the brain can be actively synthesized by or actively transported through the CP epithelial cells into the CSF. A 5 mV lumen positive voltage potential is present across CP epithelial cell membranes. This difference of electrical potential pulls sodium, chloride, and bicarbonate ions from the plasma into the CSF, creating an osmotic gradient which then drives the movement of water into the CSF.[3]
When compared to plasma, CSF has a higher concentration of sodium, chloride, and magnesium but a lower concentration of potassium and calcium.[1] Unlike plasma, CSF has only trace amounts of cells, protein, and immunoglobulins.[2] No cells can pass through the blood-CSF barrier, although small numbers of white blood cells are usually introduced to the CSF indirectly. The normal cell count of CSF is generally lower than 5 cells/ml.[1] Despite changes in blood composition and flow, the composition of CSF is kept constant, which provides a stable intraventricular environment, critical for maintaining normal neuronal function.[3]
Function
CSF assists the brain by providing protection, nourishment, and waste removal. CSF provides hydromechanical protection of the neuraxis through two mechanisms. First, CSF acts as a shock absorber, cushioning the brain against the skull. Second, CSF allows the brain and spinal cord to become buoyant, reducing the effective weight of the brain from its normal 1,500 grams to a much lesser 50 grams. The reduction in weight lessens the force applied to the brain parenchyma and cerebral vessels during mechanical injury. Another function of CSF is to maintain homeostasis of the interstitial fluid of the brain. A stable environment for brain parenchyma is imperative for maintaining normal neuronal function.
The major conduit of nutrient supply to the brain is the CP-CSF-ECSB nexus. Substrates needed by the brain are transported from the blood, through the CP, into the CSF, and then diffuse into the ECSB for transportation to their sites of action within the brain. CSF also assists in the removal of waste products of brain metabolism, such as products of peroxidation, glycosylated proteins, excess neurotransmitters, debris from the lining of the ventricles, bacteria, viruses, and otherwise unnecessary molecules. Accumulation of such unnecessary molecules, seen in aging and some neurodegenerative diseases, interferes with neuronal functioning of the brain. The great importance of CSF functioning is suggested by the disruption of cerebral physiology experienced with the disruption of the hydrodynamics or composition of CSF.[1][2][3]
Mechanism
CSF is continuously secreted with an unchanging composition, functioning to maintain a stable environment within the brain.[3] CSF is propelled along the neuroaxis from the site of secretion to the site of absorption, mainly by the rhythmic systolic pulse wave within the choroidal arteries. Lesser determinants of CSF flow are frequency of respiration, posture, venous pressure of the jugular vein, the physical effort of the subject, and time of day.[2]
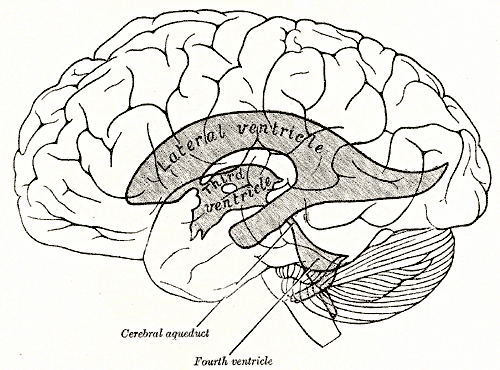
CSF is secreted by the CPs located within the ventricles of the brain, with the two lateral ventricles being the primary producers. CSF flows throughout the ventricular system unidirectionally in a rostral to caudal manner. CSF produced in the lateral ventricles would travel through the interventricular foramina to the third ventricle, through the cerebral aqueduct to the fourth ventricle, and then through the median aperture (also known as the foramen of Magendie) into the subarachnoid space at the base of the brain. Once in the subarachnoid space, the CSF begins to have a gentle multidirectional flow that creates an equalization of composition throughout the CSF. The CSF flows over the surface of the brain and down the length of the spinal cord while in the subarachnoid space. It leaves the subarachnoid space through arachnoid villi found along the superior sagittal venous sinus, intracranial venous sinuses, and around the roots of spinal nerves.
Arachnoid villi are protrusions of arachnoid mater through the dura mater into the lumen of a venous sinus. A 3 to 5 mmHg pressure gradient between the subarachnoid space and venous sinus pulls CSF into the venous outflow system through the arachnoid villi that help in its absorption. CSF may also enter into the lymphatic system via the nasal cribriform plate or spinal nerve roots. The clearance of CSF is dependent upon the posture of the subject, pressure differentials, and pathophysiology.[1][2]
Related Testing
Lumbar puncture (LP), also known as a spinal tap, is a commonly performed invasive procedure in which CSF is removed from the subarachnoid space. LP is used in the measurement of intracranial pressure and the sampling of CSF. It is commonly indicated in the evaluation of acute headaches and infection of the central nervous system. During an LP, the patient is placed in the lateral recumbent position. A sterile spinal needle is then slowly inserted between vertebrae, usually at the level of L3/4 or L4/5, into the subarachnoid space. Needle insertion may be guided by fluoroscopy or ultrasound to improve success rates and reduce the incidence of trauma.
Once CSF begins to flow through the needle, it is collected serially into four sterile tubes. Once collected, CSF can be analyzed for abnormally present or elevated CSF components, aiding in diagnosis. For example, the presence of xanthochromia, a yellow-orange discoloration of CSF caused by red blood cell degeneration, indicates the possibility of a subarachnoid hemorrhage. Elevation in concentrations of immunoglobulins, termed oligoclonal bands, may indicate the presence of a systemic infection or an autoimmune disease.
Contraindications of LP include raised intracranial pressure, bleeding disorders, and local skin infection. This procedure is relatively safe, with serious complications seldom arising. Complications of LP include infection, bleeding, radicular pain, or cerebral herniation. The most common complication is a post-LP headache with symptoms begin within 24 hours of the procedure and usually resolving by day 10.[5][6]
Clinical Significance
Hydrocephalus is a pathological condition of abnormal accumulation of CSF caused by increased CSF production, blockage of flow, or decreased absorption. The ventricles distend to accommodate elevated CSF volumes, potentially causing damage to the brain by pressing its tissue against the boney skull. Hydrocephalus may be congenital or acquired. If the flow of CSF becomes blocked throughout the ventricles, it classifies as non-communicating, or obstructive, hydrocephalus Usually the blockage is a mass such as a tumor or an abscess located within a foramen. Because CSF secretion is constant, obstruction of flow will lead to CSF build up in front of the blockage. For example, stenosis of the cerebral aqueduct, one of the most common causes of obstructive hydrocephalus, leads to enlargement of both lateral ventricles as well as the third ventricle. If the flow of CSF becomes obstructed outside the ventricles, in either the subarachnoid space or site of absorption, it classifies as communicating, or non-obstructive, hydrocephalus.
Hydrocephalus can be caused by genetic defects, infection, bleeding in the brain, trauma, or CNS tumors. Symptoms include headache, convulsions, nausea, vomiting, disturbances of vision, and mental deterioration. Diagnosis is usually through the use of imaging techniques such as ultrasound, computed tomogram (CT), or magnetic resonance imaging (MRI) scans. The most common treatment is shunt insertion, which diverts CSF away from the ventricles to an area of the body where it can be absorbed into circulation. Endoscopic third ventriculostomy, a procedure in which a hole is created in the floor of the third ventricle allowing CSF to bypass an obstruction, and cauterization of sections of CP, decreasing CSF production, are other treatment options. If left untreated, hydrocephalus yields a risk of cognitive disturbances, physical disturbances, and death.[7][8]
CSF Leak is a condition in which CSF is able to escape from the subarachnoid space through a hole in the surrounding dura. The volume of CSF lost in a leak is very variable, ranging from insignificant to very substantial amounts. If the loss of CSF is great enough, spontaneous intracranial hypotension (SIH) may occur. SIH most often presents with a positional headache caused by downward displacement of the brain due to loss of buoyancy previously provided by the CSF. Posterior neck stiffness, nausea, and vomiting are also common symptoms. The incidence of SIH is estimated to be 5/100,000 annually. Women are twice as likely to be affected and have a peak age at around 40 years.
Diagnosis is aided by typical MRI findings, such as an increase of intracranial venous volume, pituitary hyperemia, enhanced pachymeninges, and descent of the brain. Many cases of SIH resolve without any treatment. Conservative approaches such as bed rest, hydration, and increased caffeine intake may also prove to be effective; however, more drastic measures may be necessary. An epidural blood patch, where blood is injected into the spinal epidural space, may relieve CSF hypovolemic symptoms by replacing lost CSF volume with blood volume. Surgical repair of the CSF leak via a suture or metal aneurysm clip is relatively safe and usually effective in providing relief.[9]
Meningitis is a condition in which the coverings of the brain become inflamed. There are two classifications of meningitis: aseptic and bacterial. Aseptic meningitis can result from agents such as fungi, medications, and cancer metastasis, but viruses cause the majority of aseptic meningitis cases. Fever, nuchal rigidity, and photophobia are classic, presenting symptoms. Diagnosis is via an analysis of CSF obtained through LP. Viral PCR analysis of CSF is particularly helpful in diagnosing viral meningitis. Treatment is usually supportive, controlling fever and pain levels. Bacterial meningitis has a much lower incidence than aseptic meningitis but is much more serious. The incidence of bacterial meningitis has significantly dropped due to routine vaccination.
Symptoms are similar to aseptic meningitis, but clinical presentation is much more severe. Additional symptoms include altered mental status, seizures, and focal neurologic signs. Diagnosis is also possible via LP. CSF is usually cloudy in appearance, with a low glucose level, and potential positive gram stain and culture. Patients presumed to have bacterial meningitis should immediately receive broad-spectrum antibiotics to prevent clinical deterioration. After culture results return, the clinician can make adjustments to the antibiotics. Patients should also be admitted to the intensive care unit for close monitoring. Most patients with bacterial meningitis who receive appropriate treatment recover without complications.[10]
Subarachnoid Hemorrhage (SAH) is the leakage of blood into the subarachnoid space where it mixes with the CSF. Trauma is the most common cause of SAH, with 80% of nontraumatic SAHs resulting from aneurysm rupture. Other nontraumatic causes of SAH include arteriovenous malformations and vasculitis. Spontaneous SAH is a very low incidence, with only 30,000 cases worldwide annually. Ninety-seven percent of patients with SAH present with a sudden onset headache, described as a thunderclap headache and the worst of the patient's life. Other symptoms include vomiting, seizures, loss of consciousness, and death. Non-contrast head CT is useful in diagnosis. CT has high sensitivity soon after hemorrhage, but sensitivity decreases as time passes. After obtaining a negative CT, an LP should follow to rule out SAH. An LP is positive when erythrocytes are present in tubes 1 and 4, or xanthochromia is visible. Management of SAH consists of reducing risks of re-bleeding and avoiding any secondary brain injuries.[11]
Pseudotumor Cerebri Syndrome (PTCS) is a rare medical condition in which intracranial pressure is raised without the occurrence of ventriculomegaly or intracranial masses. The pathogenesis is still not well understood. The most widely accepted theory proposes decreased absorption of CSF at the arachnoid granulations or the olfactory lymphatics to be the cause. This condition has an annual incidence rate of 0.9/100,000 in the general population. Before puberty, both females and males are affected the same, but after puberty, women are affected nine times more often.
PTCS most commonly affects obese women of childbearing age. Women between the ages of 20 and 44 years and weigh 20% more than their ideal body weight have an incidence rate of 19.3/100,000. A spinal fluid examination is critical in the diagnosis of PTCS. CSF pressure is greater than 250 mm CSF in adults and 280 mm CSF in children, and adolescents are the accepted values for diagnosis PTCS. Headache is the most common presenting symptom, though there are no specific distinguishing characteristics of a PTCS headache. Occasionally asymptomatic patients present with papilledema detected during routine eye exams. Pulsatile tinnitus, transient visual obscurations, visual field defects, and visual loss are some other symptoms of PTCS.
Traditional therapy includes medications to decrease CSF secretion from the choroid plexus. Surgery is indicated for patients experiencing worsening vision caused by papilledema. Surgical options include optic nerve sheath fenestration and CSF shunting. Most patients with PTCS experience a good outcome, although a small percentage of patients continue to experience persistent headaches or blindness.[12]
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
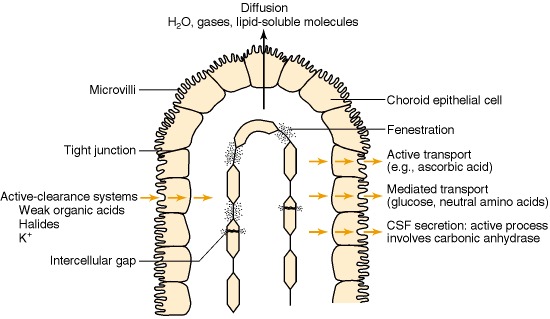
Blood—CSF barrier. The capillaries in the choroid plexus differ from those of the brain in that there is free movement of molecules across the endothelial cell through fenestrations and intercellular gaps. The blood—CSF barrier is at the choroid plexus epithelial cells, which are joined together by tight junctions. Microvilli are present on the CSF-facing surface. These greatly increase the surface area of the apical membrane and may aid in fluid secretion. Diffusion, facilitated diffusion and active transport into CSF, as well as active transport of metabolites from CSF to blood, have been demonstrated in the choroid plexus.
Laterra J, Keep R, Betz LA, et al. Blood—Cerebrospinal Fluid Barrier. In: Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27998/