Acute Pulmonary Embolism
- Article Author:
- Vrinda Vyas
- Article Editor:
- Amandeep Goyal
- Updated:
- 8/10/2020 9:09:52 PM
- For CME on this topic:
- Acute Pulmonary Embolism CME
- PubMed Link:
- Acute Pulmonary Embolism
Introduction
Pulmonary embolism (PE) occurs when there is a disruption to the flow of blood in the pulmonary artery or its branches by a thrombus that originated somewhere else. In deep vein thrombosis (DVT), a thrombus develops within the deep veins, most commonly in the lower extremities. PE usually occurs when a part of this thrombus breaks off and enters the pulmonary circulation. Very rarely, PE can occur from the embolization of other materials into the pulmonary circulation such as air, fat, or tumor cells.[1] The spectrum of PE and DVT combined is referred to as venous thromboembolism (VTE).
Etiology
Most pulmonary embolisms originate as lower extremity DVTs. Hence, risk factors for pulmonary embolism (PE) are the same as risk factors for DVT. Virchow's triad of hypercoagulability, venous stasis, and endothelial injury provides an understanding of these risk factors.
Risk factors can be classified as genetic and acquired. Genetic risk factors include thrombophilia such as factor V Leiden mutation, prothrombin gene mutation, protein C deficiency, protein S deficiency, hyperhomocysteinemia, among others. Acquired risk factors include immobilization for prolonged periods (bed rest greater than three days, anyone traveling greater than 4 hours, whether by air, car, bus, or train), recent orthopedic surgery, malignancy, indwelling venous catheter, obesity, pregnancy, cigarette smoking, oral contraceptive pill use, etc.[2][3][4][5]
Other predisposing factors for VTE include:
- Fracture of lower limb
- Hospitalization for heart failure or atrial fibrillation/flutter within the previous three months
- Hip or knee replacement
- Major trauma
- History of previous venous thromboembolism
- Central venous lines
- Chemotherapy
- Congestive heart failure or respiratory failure
- Hormone replacement therapy
- Oral contraceptive therapy
- Postpartum period
- Infection (specifically pneumonia, urinary tract infection, and HIV)
- Cancer (highest risk in metastatic disease)
- Thrombophilia
- Bed rest greater than three days
- Obesity
- Pregnancy
Cancer carries a high risk for thrombus formation and hence, PE. Pancreatic cancer, hematological malignancies, lung cancer, gastric cancer, and brain cancer carry the highest risk for VTE.[6] Infection anywhere in the body is a common trigger for VTE.[7] Myocardial infarction and congestive heart failure(CHF) increase the risk of PE. Also, patients with VTE were found to have an increased risk of subsequent stroke and myocardial infarction.[8][9]
Types of Pulmonary Embolism
It is extremely crucial to divide PE based on the presence or absence of hemodynamic stability.
Hemodynamically unstable PE (previously called massive or high-risk PE) is PE which results in hypotension (as defined by systolic blood pressure (SBP) less than 90 mmHg or a drop in SBP of 40 mm Hg or more from baseline or hypotension that requires vasopressors or inotropes), the old term "massive" PE does not describe the size of the PE but describes its hemodynamic effect. Patients with hemodynamically unstable PE are more likely to die from obstructive shock (i.e., severe right ventricular failure).
Hemodynamically stable PE is a spectrum ranging from small, mildly symptomatic or asymptomatic PE (low-risk PE or small PE) to PEs, which cause mild hypotension that stabilizes in response to fluid therapy, or those who present with right ventricle dysfunction (submassive or intermediate-risk PE), but is hemodynamically stable.
Epidemiology
The incidence of pulmonary embolism (PE) ranges from 39 to 115 per 100 000 population annually; for DVT, the incidence ranges from 53 to 162 per 100,000 people.[10] After coronary artery disease and stroke, acute pulmonary embolism is the third most common type of cardiovascular disease.[11] The incidence of PE is noted to be more in males as compared to that in females.[12] Overall, PE related mortality is high, and in the United States, PE causes 100,000 deaths annually.[12] However, the mortality rates attributable to PE can be challenging to estimate accurately because many patients with sudden cardiac death are thought to have had a thromboembolic event like PE. It is important to note that the case-fatality rates of PE have been decreasing; this might be from the improvement in diagnostic modalities and initiation of early intervention and therapies.
Pathophysiology
Pulmonary embolism occurs when clots break off and embolize into the pulmonary circulation.
Pulmonary emboli are typically multiple, with the lower lobes being involved more frequently than the upper, and bilateral lung involvement being more common.[13]
Large emboli tend to obstruct the main pulmonary artery, causing saddle embolus with deleterious cardiovascular consequences. In contrast, smaller sized emboli block the peripheral arteries and can lead to pulmonary infarction, manifested by intra-alveolar hemorrhage. Pulmonary infarction occurs in about 10% of patients.
PE leads to impaired gas exchange due to obstruction of the pulmonary vascular bed leading to a mismatch in the ventilation to perfusion ratio because alveolar ventilation remains the same, but pulmonary capillary blood flow decreases, effectively leading to dead space ventilation and hypoxemia.
Also, mediators, such as serotonin, are released, which cause vasospasm and further decreased pulmonary flow in unaffected areas of the lung. Local accumulation of inflammatory mediators alters lung surfactant and stimulates respiratory drive resulting in hypocapnia and respiratory alkalosis.[14]
In PE, pulmonary vascular resistance (PVR) increases due to the mechanical obstruction of the vascular bed with thrombus and hypoxic vasoconstriction. Pulmonary artery pressure (PAP) increases if thromboemboli occludes greater than 30% to 50% of the total cross-sectional area of the pulmonary arterial bed.
Increased PVR increases the right ventricular afterload, which impedes right ventricular outflow, which, in turn, causes right ventricular dilation and flattening or bowing of the interventricular septum. The desynchronization of the ventricles may be increased by the development of the right bundle branch block. The decreased RV outflow and concomitant RV dilation reduce left ventricular filling, thereby compromising cardiac output.[15] As a result, LV filling is reduced in early diastole, and this leads to a reduction in the cardiac output (CO), and cause systemic hypotension and hemodynamic instability. Right ventricle (RV) failure due to acute pressure overload is the primary cause of death in severe PE. Given the above pathophysiological considerations, clinical symptoms, and signs of overt RV failure and hemodynamic instability, are indicative of a high risk of early (in-hospital or 30 day) mortality.
History and Physical
A timely diagnosis of a pulmonary embolism (PE) is crucial because of the high associated mortality and morbidity, which may be prevented with early treatment. It is important to note that 30% of untreated patients with pulmonary embolism die, while only 8% die after timely therapy.[16][17] Unfortunately, the diagnosis of PE can be difficult due to the wide variety of nonspecific clinical signs and symptoms in patients with acute PE.
The most common symptoms of PE include the following: dyspnea, pleuritic chest pain, cough, hemoptysis, presyncope, or syncope. Dyspnea may be acute and severe in central PE, whereas it is often mild and transient in small peripheral PE. In patients with preexisting heart failure or pulmonary disease, worsening dyspnea may be the only symptom. Chest pain is a frequent symptom and is usually caused by pleural irritation due to distal emboli causing pulmonary infarction.[18] In central PE, chest pain may be from underlying right ventricular (RV) ischemia and needs to be differentiated from an acute coronary syndrome or aortic dissection.
Less common presentations include arrhythmias (e.g., atrial fibrillation), syncope, and hemodynamic collapse.[19] Hemodynamic instability is a rare but essential form of clinical presentation, as it indicates central or extensive PE with severely reduced hemodynamic reserve. Syncope may occur and may be associated with a higher prevalence of hemodynamic instability and RV dysfunction.[20] It is essential to recognize that patients with large PE may, at times, be asymptomatic or have mild symptoms. Many times, PE may be asymptomatic or discovered incidentally during diagnostic workup for another disease.
Apart from symptoms of PE, it is crucial to look for the risk factors for venous thromboembolism (VTE) to determine the clinical probability of a PE.
On examination, patients with PE might have tachypnea and tachycardia, which are common but nonspecific findings. Other examination findings include calf swelling, tenderness, erythema, palpable cords, pedal edema, rales, decreased breath sounds, signs of pulmonary hypertension such as elevated neck veins, loud P2 component of second heart sound, a right-sided gallop, and a right ventricular parasternal lift might be present on examination.
PE is a well-recognized cause of sudden cardiac arrest (8%).[21] A massive PE leads to an acute right ventricular failure, which presents as jugular venous distension, parasternal lift, third heart sound, cyanosis, and shock. If a patient with PE who has tachycardia on presentation develops sudden bradycardia or develops a new broad complex tachycardia (with right bundle branch block), providers should look for signs of right ventricular strain and possible impending shock. PE should be suspected in anyone who has hypotension with jugular venous distension wherein acute myocardial infarction, pericardial tamponade, or tension pneumothorax has been ruled out.[22]
Evaluation
Diagnostic Workup
Arterial Blood Gas (ABG) Analysis
Unexplained hypoxemia with a normal chest radiograph should raise the clinical suspicion for pulmonary embolism (PE). Widened alveolar-arterial gradient for oxygen, respiratory alkalosis, and hypocapnia are commonly seen findings on ABG, as a pathophysiological response to pulmonary embolism. It is important to note that hypercapnia, respiratory, or lactic acidosis is not common but can be present in patients with massive PE associated with obstructive shock and respiratory arrest.
Brain Natriuretic Peptide (BNP)
Elevated BNP has limited diagnostic importance in patients suspected of having PE.[23] Right ventricle pressure overload because of acute PE is associated with more myocardial stretch, which then releases B-type natriuretic peptide (BNP) and N-terminal (NT)-proBNP. Thus, the levels of natriuretic peptides in blood reflect the severity of RV dysfunction in acute PE.[24]
Troponin
Serum troponin I and T levels are beneficial prognostically but not diagnostically.[25][26] As markers of right ventricular dysfunction, troponin levels are elevated in 30 to 50 percent of patients with moderate to large PE and are linked to clinical deterioration and death after PE.[27]
D-dimer
D-dimer levels are elevated in plasma whenever there is an acute thrombotic process in the body because of the activation of coagulation and fibrinolysis pathways at the same time. D-dimer testing has high negative predictive value; hence, a normal D-dimer level makes acute PE or DVT unlikely. But since the positive predictive value of elevated D-dimer levels is low, D-dimer testing is not useful for confirmation of PE. As many D-dimer assays are available, providers should become aware of the diagnostic performance of the test used in their clinical setting. The quantitative enzyme-linked immunosorbent assay (ELISA) has a diagnostic sensitivity of at least 95%. It can be used to exclude the diagnosis of PE in patients with either low or intermediate pretest probability. A negative ELISA D-dimer, along with low clinical probability, can exclude PE without further testing in approximately 30% of suspected patients.
The specificity of D-dimer decreases steadily with age to approximately 10% in patients greater than 80 years of age. The use of age-adjusted cut-offs for patients older than 50 may improve the performance of D-dimer testing in the elderly. In one study, the use of the age-adjusted cut-off instead of the standard D-dimer cut-off of 500 ng/mL or more increased the number of patients in whom the possibility of PE could be ruled out from 6.4% to 30%, without additional false-negative findings.[28]
The formula is age (years) x 10 mcg/L for patients more than 50 years of age. Example: Patient age 75 = age-adjusted d-dimer of 750 mcg/L.
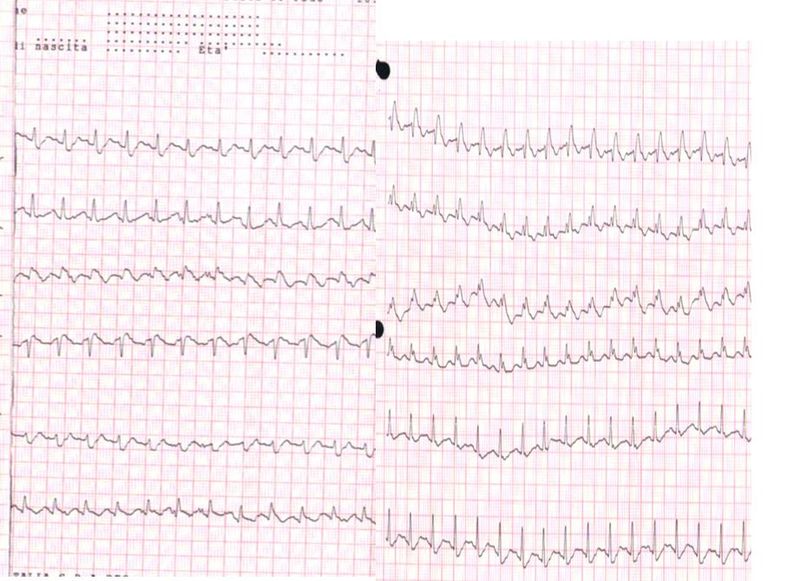
Electrocardiography (ECG)
ECG abnormalities, in patients with suspected PE, are nonspecific.[29] The most common ECG findings in PE are tachycardia and nonspecific ST-segment and T-wave changes, S1Q3T3 pattern, right ventricular strain, and new incomplete right bundle branch block are uncommon.
Chest Radiograph (CXR)
In PE, CXR is usually normal or might show nonspecific abnormalities such as atelectasis or effusion. It helps to rule out alternative diagnoses in patients presenting with acute dyspnea.
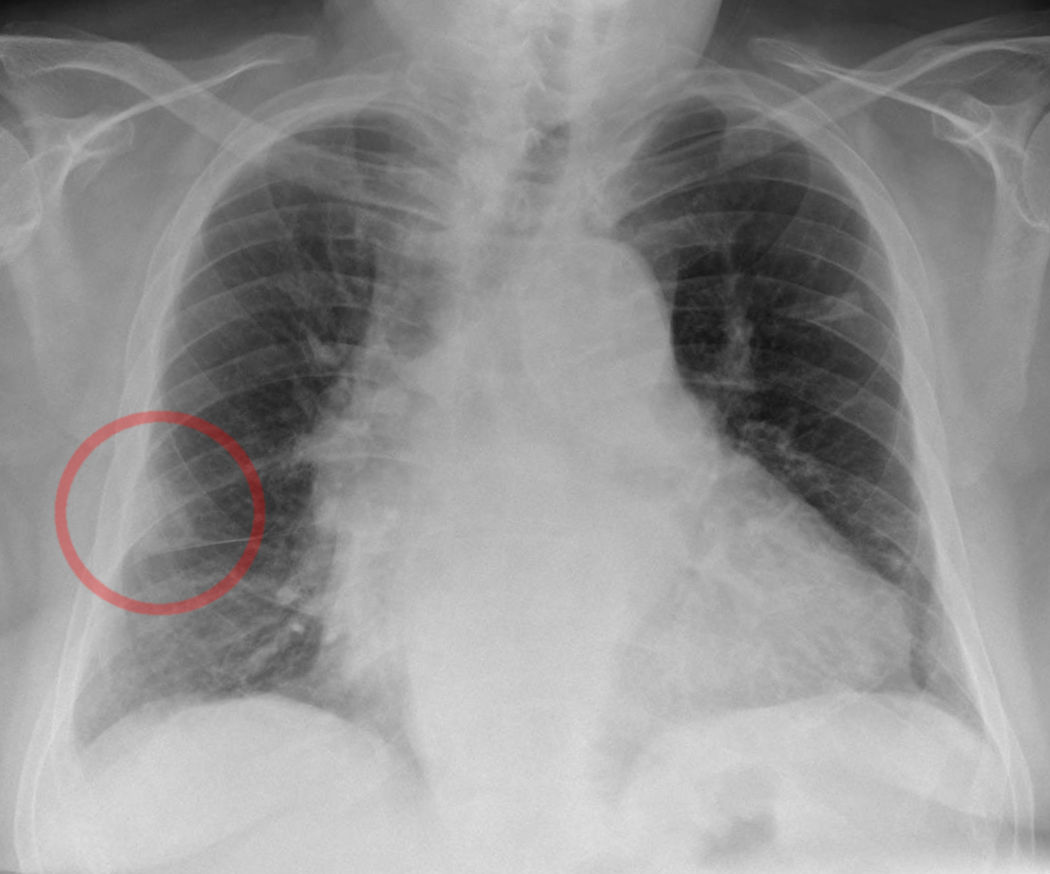
Hampton's hump is a shallow, hump-shaped opacity on CXR in the periphery of the lung, with its base lying against the pleural surface and hump towards the hilum (Figure 1). Westermark's sign is the sharp cut-off of pulmonary vessels with distal hypoperfusion in a segmental distribution within the lung; both of these findings are rare but specific for acute PE.[30] 'Westermark sign' may be seen in up to 2% of the cases. This finding is a result of a combination of dilation of the pulmonary artery proximal to the thrombus and the collapse of the distal vasculature (figure 2).
Computed Tomographic Pulmonary Angiography (CTPA)
Multidetector CTPA is the diagnostic modality of choice for patients with suspected PE. It allows appropriate visualization of the pulmonary arteries down to the subsegmental level.[31] The PIOPED (Prospective Investigation On Pulmonary Embolism Diagnosis) II study showed a sensitivity of 83% and a specificity of 96% for CTPA in PE diagnosis.[32] PIOPED II also highlighted the pretest clinical probability influence on the predictive value of CTPA. A normal CTPA had a high negative predictive value for PE 96% and 89% in patients with a low or intermediate clinical probability, respectively, but its negative predictive value was only 60% if the pretest probability was high. In contrary to this, the positive predictive value of a positive CTPA was high (92% to 96%) in patients with an intermediate or high clinical probability, but much lower (58%) in patients with a low pretest likelihood of PE.[32] Therefore, providers should consider further testing in case of discordance between clinical judgment and the CTPA result.
The present data suggest that a negative CTPA result is adequate for the exclusion of PE in patients who have a low or intermediate clinical probability. Besides, it remains controversial whether patients with a negative CTPA and a high clinical probability should be further investigated.
CTPA may be relatively contraindicated in moderate to severe iodinated contrast allergy or renal insufficiency (eGFR less than 30 mL/min per 1.73-meter square). The risk of these contraindications must be measured against the clinical significance of performing the CTPA examination and the availability of other imaging modalities (e.g., V/Q scan). If clinically feasible, CTPA should be postponed for premedication for a history of allergy or intravenous hydration for renal insufficiency.
CTPA can detect RV enlargement and other indicators of RV dysfunction. Enlarged RV has prognostic value, and it is supported by the results of a prospective multicentre cohort study in 457 patients.[33] In that study, RV enlargement (RV/LV ratio ≥0.9) was a strong and independent predictor of a severe in-hospital outcome, both in the overall population and in hemodynamically stable patients.
Lung Scintigraphy
The planar ventilation/perfusion scan (V/Q scan) is an established diagnostic test for suspected PE. V/Q scanning is mostly performed for patients in whom CTPA is contraindicated or inconclusive, or when additional testing is needed. A normal chest radiograph is usually required before V/Q scanning. Scans performed on patients with abnormal chest radiographs are most likely to be false positives because the images do not appear normal or low probability of PE in such patients.
For those with a normal chest radiograph, V/Q scanning remains the test of choice for the diagnosis of PE in pregnancy. Other groups of patients include those who have a history of contrast medium-induced anaphylaxis and patients with severe renal failure.[34]
Planar lung scan results are frequently classified into three-tiers: normal scan (excluding PE), high-probability scan (considered diagnostic of PE in most patients), and nondiagnostic scan.[34][35] Multiple studies have suggested that it is safe to withhold anticoagulant therapy in patients with a normal perfusion scan.[36] An analysis from the PIOPED II study advocated that a high-probability V/Q scan can confirm PE. However, the positive predictive value of a high-probability V/Q scan is not enough to confirm the PE diagnosis in patients with a low clinical probability.[37] The high frequency of nondiagnostic scans is a limitation because they indicate the necessity for further diagnostic testing.
Pulmonary Angiography
In pulmonary angiography, contrast is injected via a catheter introduced into the right heart under fluoroscopy, which was the gold standard in the past for the diagnosis of PE. The diagnosis of acute PE is made on the evidence of a thrombus either as amputation of a pulmonary arterial branch or filling defect.[38] With the widespread emergence of CTPA, pulmonary angiography is infrequently used and reserved for rare circumstances for patients with a high clinical probability of PE, in whom CTPA or V/Q scanning is nondiagnostic. Pulmonary angiography seems to be inferior to CTPA, and its results are operator dependent and highly variable.[39] Therefore, catheter-based pulmonary angiography is performed in patients who need therapeutic benefit since it helps with diagnosis as well as therapeutic interventions aimed at clot lysis.
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) has been assessed for several years regarding suspected PE. However, the results of large-scale studies show that this technique, although promising, is not recommended as a first-line test for the diagnosis of PE due to its low sensitivity, low availability in most emergency settings, and the high proportion of inconclusive MRA scans.[40] But it may be an imaging option for diagnosis of PE in patients in whom neither CTPA nor V/Q scan can be performed. Potential advantages include no exposure to radiation. MR pulmonary angiography was studied prospectively in 371 adults with suspected PE. Among the 75 percent of patients who had technically adequate images, MRPA alone showed a sensitivity and specificity of 78 percent and 99 percent, respectively.[40] Among the 48 percent of patients with technically adequate images, MR pulmonary angiography and MR pulmonary venography have shown a sensitivity and specificity of 92 percent and 96 percent, respectively.
Echocardiography
Transthoracic echocardiography can very rarely diagnose PE definitively when the thrombus is visualized in the proximal pulmonary arteries. The diagnosis of PE on echocardiography is supported by the presence of clot in the right heart or new right heart strain, especially in hemodynamically unstable patients with suspected PE wherein echocardiogram may be useful to establish a possible diagnosis and justify the emergency use of thrombolytic therapy.
There are significant considerations with using echocardiography to establish a diagnosis of PE. Given the peculiar shape of the RV, there is no single echocardiographic parameter that gives quick and accurate information on RV size or function. That is why echocardiographic criteria for the diagnosis of PE have varied between different studies. Because of the negative predictive value of 40% to 50%, a negative result cannot exclude PE.[41][42] On the other hand, signs of RV overload or dysfunction may also be present without acute PE, and may be due to coexisting cardiac or respiratory disease.[43]
RV dilation is seen in 25% or more of patients with PE on echo and is useful for risk stratification of the disease.[44] More specific echocardiography findings confer a high positive predictive value for PE, even in the presence of preexisting cardiorespiratory illness. This includes, the combination of a pulmonary ejection acceleration time (measured in the RV outflow tract) less than 60 ms with a peak systolic tricuspid valve gradient less than 60 mmHg ('60/60' sign), or McConnell sign (with depressed contractility of the RV free wall compared to the RV apex), is suggestive of PE.[45]. An RV/LV diameter ratio 1.0 or more and tricuspid annular plane systolic excursion (TAPSE) less than 16 mm are the findings for which an association with unfavorable prognosis has most frequently been reported.[46]
Compression Ultrasonography (US)
PE originates from a lower limb DVT in a majority of patients, and only rarely from upper-limb DVT (mostly following venous catheterization). In one study, DVT was found in 70% of patients with proven PE.[47] Compression US has a sensitivity of more than 90% and a specificity of about 95% for proximal symptomatic DVT.[48] A finding of proximal DVT in patients suspected of having PE is considered sufficient to warrant anticoagulant treatment without further testing.[49] It is important to note that, due to the low sensitivity of compression ultrasonography, it is reserved for patients in whom definitive imaging (e.g., CTPA, V/Q scanning) is contraindicated or indeterminate.[50]
Wells criteria and Geneva score are scoring systems most commonly used to estimate the pretest probability of having a PE. This allows the classification of patients with suspected PE into categories of clinical or pretest probability, based on which the diagnostic tests are chosen and interpreted.
The Revised Geneva Clinical Prediction Rule
Items/Clinical decision rule points (Original version)(Simplified version)
- Previous PE or DVT-3/1
- Heart rate
- 75–94 beats per minute-3/1
- ≥95 beats per minute-5/2
- Surgery or fracture within the past month-2/1
- Hemoptysis-2/1
- Active cancer-2/1
- Unilateral lower-limb pain-3/1
- Pain on lower-limb deep palpation and unilateral edema-4/1
- Age >65 years-1/1
Clinical Probability
Three-level score
- Low/0–3/0–1
- Intermediate/4–10/2–4
- High/≥11/≥5
Two-level Score
- PE-unlikely/0–5/0–2
- PE-likely/≥6/≥3
Wells Criteria and Modified Wells Criteria
Items/Scores
- Clinical symptoms of DVT-3.0
- Other diagnoses less likely than pulmonary embolism-3.0
- Heart rate >100 beats per min-1.5
- Immobilization for three or more days or surgery in the previous four weeks-1.5
- Previous history of DVT-PE1.5
- Hemoptysis-1.0
- Malignancy-1.0
Probability/Score
Traditional Clinical Probability Assessment (Wells criteria)
- High/>6.0
- Moderate/2.0 to 6.0
- Low/<2.0
Simplified Clinical Probability Assessment (Modified Wells Criteria)
- PE likely/>4.0
- PE unlikely/≤4.0
Since symptoms of PE are very nonspecific, The Pulmonary Embolism Rule-out Criteria (PERC) was developed for emergency department patients to select patients whose likelihood of having PE is so low that diagnostic workup should not even be initiated.[51] They constitute variables significantly associated with the absence of PE.
The PERC rule has eight criteria:
• Age <50 years
• Heart rate <100 beats per minute
• Oxyhemoglobin saturation ≥95 percent
• No hemoptysis
• No estrogen use
• No prior DVT or PE
• No unilateral leg swelling
• No surgery/trauma requiring hospitalization within the preceding four weeks
Patients having a low probability of PE who fulfill all eight criteria, the likelihood of PE is sufficiently low that further testing is not indicated.
PERC is only valid in clinical settings with a low prevalence of PE (<15 percent).[52] In hospital settings with a higher prevalence of PE (>15 percent), the PERC-based approach has substantially weaker predictive value.[53] Therefore, it should not be used in patients with an intermediate or high suspicion for PE or for inpatients suspected as having PE.
Diagnostic Approach to Hemodynamically Stable Patients with Suspected Pulmonary Embolism
For most patients with suspected PE who are hemodynamically stable, an approach that combines clinical and pretest probability assessment, D-dimer testing, and definitive diagnostic imaging is usually applied.
For a Patient with a Low Probability of PE (Wells Score <2)
If PERC criteria are fulfilled, there is no need for further testing, and PE can be excluded. If PERC criteria are not met, then D-dimer should be obtained. If D-dimer is negative(<500 ng/ml), PE can be ruled out if D-dimer is positive (>500 ng/ml) in patients age <50 or high after age-adjusted D-dimer value, CT pulmonary angiography should be performed. If CTPA is inconclusive or contraindicated, V/Q scan should be performed.
For a patient with intermediate probability of PE (Wells score 2 to 6)
Measure D-dimer levels, if negative, PE can be excluded. If positive, then CTPA is done. If CTPA is inconclusive or contraindicated, V/Q scan should be performed.
For a Patient with a High Probability of PE (Wells Score >6)
CTPA should be performed emergently. Feasibility requires adequate scanner technology, and the patient must be able to lie flat, to cooperate with exam breath-holding instructions, have a body habitus that can fit into the scanner, and no contraindications for iodinated contrast. If inconclusive or not feasible, perform a V/Q scan. V/Q scan could be normal, ruling out PE. It could also have resulted as a "high probability for PE," which would be diagnostic of PE if V/Q scan results as intermediate probability, further testing with lower extremity compression ultrasonography with Doppler is appropriate.
For patients who are hemodynamically unstable and in whom definitive imaging is unsafe, bedside echocardiography or venous compression ultrasound may be used to obtain a presumptive diagnosis of PE to justify the administration of potentially life-saving therapies.
Treatment / Management
A) Initial Management
1) The Supportive Measures
The initial approach to patients with pulmonary embolism (PE) should focus on supportive measures.
Supplemental oxygen is indicated in patients with oxygen saturation <90%. Mechanical ventilation (non-invasive or invasive) should be utilized in unstable patients, but providers should be mindful of the adverse hemodynamic effects of mechanical ventilation.
Acute RV failure is the leading cause of death in patients with hemodynamically unstable PE. Aggressive volume resuscitation in such patients can over distend the RV, worsen ventricular interdependence, and reduce cardiac output (CO). Hence, in patients with massive PE, intravenous fluid resuscitation should be tried only in patients with collapsible IVC/intravascular depletion. Vasopressors might be needed for hemodynamic support.
Mechanical cardiopulmonary support devices, such as extracorporeal membrane oxygenation (ECMO), may be used in hemodynamically unstable patients with pulmonary embolism.
2) Anticoagulation
It is vital to remember that the mainstay of treatment of acute PE is anticoagulation.
It is important to note that either low-molecular-weight heparin (LMWH) or fondaparinux or unfractionated heparin (UFH) can be used for anticoagulation in acute PE. LMWH and fondaparinux are preferred since they have a less incidence of inducing major bleeding and heparin-induced thrombocytopenia.[54][55] UFH is usually only used in patients with hemodynamic instability in whom primary reperfusion treatment might be required, or in patients with renal impairment. Newer oral anticoagulants (NOACs) and vitamin K antagonists(VKA) can also be used for anticoagulation in PE.
For patients with suspected PE, the treatment is stratified according to the type of PE ( whether it is hemodynamically stable or unstable PE) and according to the suspicion of PE in an individual patient. Patients are classified into low, intermediate, or high suspicion for PE based on either revised Geneva or Wells score.
Hemodynamically Stable Patients:
Patients with a high clinical suspicion for PE, anticoagulation is started even before diagnostic imaging is obtained.
For patients with a low clinical suspicion for PE, if diagnostic imaging can be performed within 24 hours, then wait for imaging to establish a definitive diagnosis before starting treatment with anticoagulation.
For patients with an intermediate clinical suspicion for PE, if diagnostic imaging can be performed within 4 hours, then wait for imaging to establish a definitive diagnosis before starting treatment with anticoagulation.
For patients in whom anticoagulation is contraindicated, IVC filter placement should be considered once the diagnosis of PE is confirmed.
Hemodynamically Unstable Patients:
Patients with a high clinical suspicion for PE who are hemodynamically unstable, emergent CTPA, portable perfusion scanning, or bedside transthoracic echocardiography should be performed whenever possible. Primary reperfusion treatment, usually, thrombolysis, is the treatment of choice for patients with hemodynamically unstable acute PE. Surgical pulmonary embolectomy or percutaneous catheter-directed therapy are alternative reperfusion options in patients with contraindications to thrombolysis. Following reperfusion treatment and hemodynamic stabilization, patients recovering from high-risk PE can be switched from parenteral to oral anticoagulation.
3) Reperfusion Strategies
Thrombolysis:
Thrombolysis has shown an effective reduction in pulmonary artery pressure and resistance in patients with PE when compared with UFH alone; these improvements are assessed by a decrease in RV dilation on echocardiography.[56][57] Thrombolysis is preferred when therapy can be instituted within 48 hours of symptom onset, but it has still shown benefit in patients whose symptoms began less than 14 days ago.[58] A meta-analysis suggested a significant reduction in mortality and recurrent PE with the use of thrombolytics.[59]
Pulmonary Embolism Thrombolysis (PEITHO) trial identified the benefits of thrombolysis in hemodynamically stable patients with intermediate-risk PE.[60] It demonstrated that thrombolysis was associated with a significant reduction in the risk of hemodynamic decompensation or collapse, but it also showed an increased risk of severe bleeding with thrombolytics.[60][61]
Absolute contraindications to thrombolysis include any prior intracranial hemorrhage, known structural intracranial cerebrovascular disease (e.g., arteriovenous malformation), known malignant intracranial neoplasm, ischemic stroke within three months, suspected aortic dissection, active bleeding or bleeding diathesis, recent surgery encroaching on the spinal canal or brain, and recent significant closed-head or facial trauma with radiographic evidence of bony fracture or brain injury.
Catheter-Directed Treatment:
Involves the insertion of a catheter into the pulmonary arteries, which is then used for ultrasound-assisted thrombolysis, suction embolectomy, rotational embolectomy, thrombus aspiration, or combining mechanical fragmentation with pharmacological catheter-directed thrombolysis. Different studies have shown a success rate of up to 87% for catheter-directed therapies.[62][63] Catheter-assisted embolectomy techniques carry the inherent risk of perforating the pulmonary arteries, leading to massive hemoptysis or cardiac tamponade. These complications are rare but fatal.
Surgical Embolectomy
It is usually indicated in a patient with hemodynamically unstable PE in whom thrombolysis (systemic or catheter-directed) is contraindicated, or in patients who have failed thrombolysis.[64][65][66] Thrombolysis or surgical embolectomy, there was no difference in mortality between the two, but the thrombolysis group had a higher risk of stroke and re-intervention.
Vena Cava Filters
These block the path of travel of emboli and prevent them from entering the pulmonary circulation. Filters are indicated in patients with venous thromboembolism who have an absolute contraindication to anticoagulants, and in patients with recurrent VTE despite anticoagulation. Retrievable filters are preferred, such that once the contraindication has resolved, the filter can be removed, and patients should be anticoagulated. This is because, the Prevention of Recurrent Pulmonary Embolism by Vena Cava Interruption (PREPIC) study showed that the insertion of a permanent vena cava filter was associated with a significant reduction in the risk of recurrent PE and a substantial increase in the risk of DVT, without a remarkable difference in the risk of recurrent VTE or death.[67]
B) Chronic Treatment and Prevention of Recurrence
The aim of anticoagulation after the acute management of PE is to complete the treatment of the acute episode and also prevent the recurrence of VTE over the long-term. Clinical trials have assessed various durations of anticoagulant therapy with vitamin K antagonists (VKAs) for VTE.[68][69][70] The findings of these studies have concluded the following points. First, all patients with PE should receive three or more than three months of anticoagulant treatment. Second, after the anticoagulant treatment is stopped, the risk of recurrence is expected to be similar if anticoagulants are stopped after 3-6 months compared with longer treatment periods (e.g., 12-24 months). Third, extended oral anticoagulant treatment reduces the risk of recurrent VTE by ≤90%, but the risk of bleeding partially offsets this benefit. Oral anticoagulants are highly efficient in preventing recurrent VTE at the time of treatment, but after the discontinuation of treatment, they do not eliminate the risk of subsequent recurrence.[68][68][68] It is important to note that about 30% of PEs are unprovoked. Unprovoked PE ( PE in the absence of an identifiable risk factor) is associated with a two- to three-fold increase in the risk of recurrence compared to patients who had a provoked PE.[71] Patients with persistent risk factors (e.g., cancer or elevated antiphospholipid antibodies) have a higher rater of recurrence than those with transient risk factors (e.g., immobilization, surgery, or trauma).[72]
In conclusion, the optimal duration of anticoagulation remains uncertain and has to be considered on a case to case basis. A minimum of 3 months is usually recommended, but a more extended period is required if the PE was unprovoked or if there are persistent risk factors.[71] This need for longer anticoagulation should be assessed at the end of 3 months by considering the patient's bleeding risk. Those with a high bleeding risk can limit therapy to three months.
Special considerations are required for patients with active cancer, given their increased risk for a VTE event. Hence, cancer patients should receive an extended duration of anticoagulation if their bleeding risk remains acceptable ( low or moderate bleeding risk). For cancer patients with PE, LMWH, and DOACs (apixaban, rivaroxaban) are preferred over VKA.[73]
Differential Diagnosis
Since pulmonary embolism has a very heterogeneous clinical presentation ranging from dyspnea to sudden cardiac arrest. The differential diagnosis of PE is extensive and includes:
- Acute coronary syndrome
- Stable angina
- Acute pericarditis
- Congestive heart failure
- Malignancy
- Cardiac arrhythmias
- Pneumonia
- Pneumonitis
- Pneumothorax
- Vasovagal syncope
Prognosis
Shock and right ventricular dysfunction confer a poor prognosis and predict mortality in patients diagnosed with PE.[52] Patients with pulmonary embolism and a coexisting deep vein thrombosis (DVT) are also at an increased risk for death. Several prognostic models have been designed, the Pulmonary Embolism Severity Index (PESI) and the simplified PESI (sPESI) are the most commonly used. PESI score predicts 30-day mortality in patients with an established diagnosis of PE.[74] The principal strength of the PESI and sPESI lies in the identification of patients at low risk for 30-day mortality (PESI classes I and II).
Original and simplified Pulmonary Embolism Severity Index
- Parameter- original version[74]/simplified version[75]
- Age-1 point (if age >80 years)
- Male sex- +10 points/ –
- Cancer- +30 points/1 point
- Chronic heart failure- +10 points/1 point
- Chronic pulmonary disease- +10 points/1 point
- Pulse rate ≥110 b.p.m- +20 points/1 point
- Systolic BP <100 mmHg- +30 points/1 point
- Respiratory rate >30 breaths per min- +20 points/–
- Temperature <36°C- +20 points/–
- Altered mental status- +60 points/–
- Arterial oxyhaemoglobin saturation <90%- +20 points/1 point
Risk stratification in PESI
- Class I: Points less than or equal to 65; low 30-day risk of mortality from 1 to 6 percent.
- Class II: Points 66–85; low mortality risk from 1.7 to 3.5%
- Class III: Points 86–105; moderate mortality risk from 3.2 to 7.1 percent.
- Class IV: Points 106–125; high mortality risk from 4.0 to 11.4%.
- Class V: Points more than 125; very high mortality risk from 10.0 to 24.5%.
Risk stratification in sPESI
If 0 points then 30-day mortality risk 1.0%
If one or more than one point(s) then 30-day mortality risk 10.9%
Complications
The major complications associated with pulmonary embolism (PE) include the following:
- Recurrent thromboembolism
- Chronic thromboembolic pulmonary hypertension
- Right heart failure
- Cardiogenic shock
PE, if left untreated, is associated with mortality of up to 30 percent. Studies have also suggested an increased risk of stroke, thought to be due to paradoxical embolism via a patent foramen ovale (PFO) in patients with acute PE.[76]
1) Recurrent Thromboembolism
In the one to two weeks following diagnosis, patients may deteriorate and experience recurrence. Inadequate anticoagulation is the most common reason for recurrent venous thromboembolism while on therapy.
2) Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
The development of persistent or progressive dyspnea, particularly during the first three months to two years of diagnosis, should prompt the provider to investigate for the development of CTEPH (affects up to 5 percent of patients). Follow-up computed tomography, ventilation-perfusion scans, or echocardiography should be performed in patients who remain persistently symptomatic months to years after an acute PE. In CTEPH, these modalities demonstrate pulmonary hypertension.
On the V/Q scan, patients with CTEPH generally have at least one segmental or more significant mismatched ventilation-perfusion defects. For those patients with evidence of CTEPH on V/Q lung scanning, right heart catheterization and pulmonary angiography are indicated to confirm pulmonary hypertension, quantify the degree of pulmonary hypertension, exclude competing diagnoses, define the surgical accessibility of the obstructing thrombotic lesions, and confirm that an acceptable component of the elevated pulmonary vascular resistance is due to surgically accessible disease and not from distal obstruction or a secondary arteriopathy. For all patients with CTEPH, lifelong anticoagulant therapy is recommended. Also, early referral for evaluation for pulmonary thromboendarterectomy is highly recommended.
Pearls and Other Issues
A timely diagnosis of pulmonary embolism (PE) is crucial because of the high associated mortality and morbidity, which may be prevented with early treatment. Patients should be made aware of the signs and symptoms of VTE and PE since the incidence of recurrent thromboembolism is high. It is important to note that 30% of untreated patients with pulmonary embolism die, while only 8% die after timely therapy.
Enhancing Healthcare Team Outcomes
Some hospitals are establishing multidisciplinary pulmonary embolism response teams (PERTs) to facilitate prompt diagnosis and timely treatment of patients with pulmonary embolism. A PERT is a treatment model composed of providers from different specialties involved in the treatment of PE, including pulmonary, critical care, cardiology, and cardiothoracic surgery, among others. The establishment of PERT has proven effective in multiple studies by improving communication and coordinating treatment efforts among providers.[77]
(Click Image to Enlarge)
Contribute by Wikimedia Commons, Goslar T, Podbregar M. (CC by 2.0) https://creativecommons.org/licenses/by/2.0/deed.en