Aortic Valve Repair
- Article Author:
- Talha Ahmed
- Article Editor:
- Yana Puckett
- Updated:
- 7/31/2020 3:19:05 PM
- For CME on this topic:
- Aortic Valve Repair CME
- PubMed Link:
- Aortic Valve Repair
Introduction
Aortic valve functional unit is a central outflow tract of the left side of the heart. The operational unit of the left outflow tract consists of aortic valve cusps, commissures, annulus, and sinuses at the root of the aorta.[1] This functional unit of the left ventricular outflow tract is responsible for maintaining the laminar flow of blood after ejection from the left ventricle. Aortic valve pathologies can have a severe generalized impact on the body based on the perfusion deficit. Aortic valve insufficiency is mostly due to the underlying shear force causing stretch, dilation, rupture of the valve.[2]
Moreover, conditions of the aortic root, including coarctation, dissection, and aneurysm, can increase the aortic valve pathology due to increase shear force of blood at the aortic valve disrupting the laminar flow.[3] Aortic valve pathologies can be managed by repair or replacement. Aortic valve replacement with a biological or prosthetic valve has been the cornerstone of the management of aortic valve pathologies. However, aortic valve repair has been the topic of debate as compared to replacement in specific subsets of patients. Over the last decade, aortic valve repair and preservation gained exceptional significance in the treatment of aortic root disease with and without insufficiencies. Successful aortic valve repair and preservation methods have been performed worldwide with a prevalence of procedures, less than 2%, as it needs excellent expertise.[4]
The prevalence of procedure performed is low as compared to mitral valve repair as calcific aortic stenosis is the most common valvular pathology seen, which has less success. As compared to repair, aortic valve replacement seems to be relatively simpler, but it has the downside of causing long term complications of structural deterioration, restenosis, infection, and bleeding due to anticoagulation.[5][6] Therefore, aortic valve preservation procedures have become a reasonable alternative in preventing complications of replacement by maintaining nearby normal anatomy and physiology of the aortic valve functional unit.
Anatomy and Physiology
In order to understand the valve repair and preservation procedure, it is crucial to understand the surgical anatomy of the aortic valve unit. The aortic valve functional unit consists of the ventriculoaortic junction (AVJ), sinotubular junction (STJ), aortic leaflets, and sinuses of the aortic valve. The origin of the aorta is close to the cardiac valve, provide a bridge between the left ventricle and the systemic circulation. The internal part of the aortic root contains the leaflets, interleaflet triangle, and commissures. Typically, the coaptation height is at the mid-level between the STJ and AVJ.[7] The aortic root is the basal attachments of the aortic valve leaflets to the sinotubular junction. Henle was the first person to define the arterial root instead of the arterial ring. The aortic root bulges to form the three sinuses. Two of the aortic sinuses give origin to the main coronary arteries. The last sinus is furthest from the pulmonary trunk and is a non-coronary aortic sinus. However, the sinuses are named anterior (for right coronary), left posterior (for left coronary), and right dorsal (for non-coronary), anatomically.[8] Parts of the aortic valve described below.
1. Ventriculoaortic Junction (VAJ): This elliptical junction marks the transition of left ventricle outflow and aortic root. The mean diameter of VAJ size is 23 mm in the adult population with standard AV.[9][10] For anatomical understanding, VAJ is the zone where ventricle muscles terminate and start to connect with aortic sleeve wall, and for surgical knowledge, VAJ depicts leaflet hinge-lines.[11] The crown shape of the aortic cusps represents the line of insertion. The virtual basal ring/annulus is the line passing through the nadir of each adjacent cusps and defined as a commissure. Three-dimensional crown-shaped attachment of the cusps, the aortic annulus, is the function unit. The annulus intraluminal region Joins the nadir of the three cusps. The circumferential plane of annulus joins the three commissures and represent sinotubular junction. Moreover, for understanding the extern aortic dissection can extend to the basal ring on the posterior portion of the root that is left coronary sinus/left commissure and usually don't extend to the right anterior portion coronary sinus/right commissure.[12][13]
2. Sinutubular Junction (STJ): It is a part of the aortic annulus circumferential plane that joins the three commissures. The diameter of STJ is 5mm more than the base of the valve, creating a 1.3 ration between the sinotubular junction and the virtual basal annulus.[12]
3. Aortic Leaflets: The cusp size is crucial to rule out prolapse or deficient tissue for reconstruction. The intraoperative measurement of cusp size/height is cumbersome. A geometric' height of a cusp is the most significant distance between the insertion of the aorta and the free center margin of the cusp. The effective height of a cusp is measure from the basal plane to the central coaptation. A successful aortic valve repair on a cutoff margin of the retracted cusps. Retracted cusps defined as having geometric height<19 mm in bicuspid valves and <16 mm in tricuspid valves.[14]
4. Aortic Sinuses of Valsalva: The top of the central valve leaflet forms a stable region by the transition of the elliptical base to circular at the STJ.
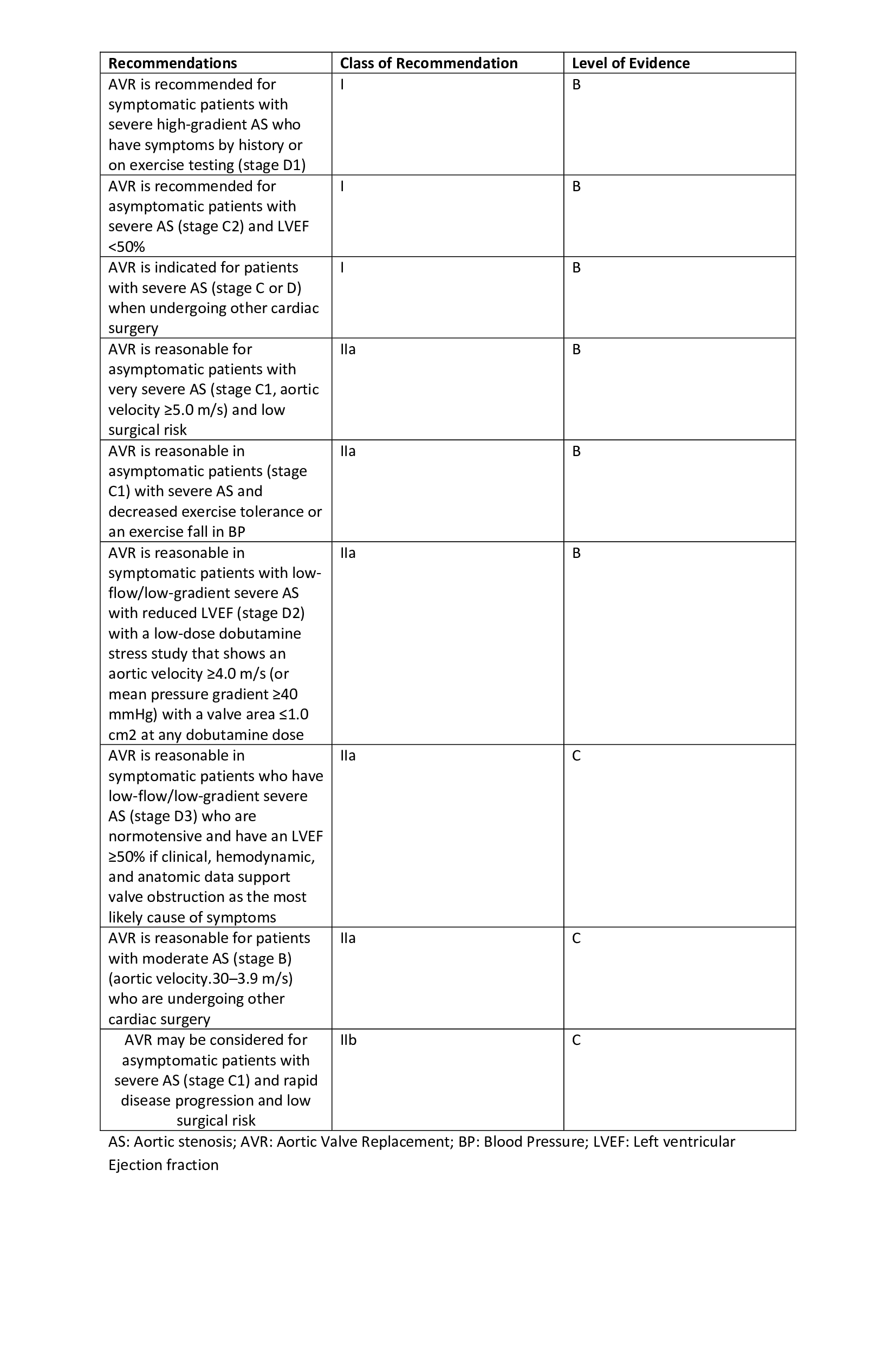
Indications
Currently, the indications of aortic valve repair are the same as that of valve replacement with a benefit of low perioperative complications.[15] Indications include patients with symptomatic or asymptomatic aortic insufficiency with significant left ventricular dilation (LV end-systolic diameter > 50 mm and LV end-diastolic diameter > 70 mm) or left ventricular dysfunction.[16] Another indication is to perform valve repair in aortic root disease or ascending aorta with valvular pathology.[17][18] Further, experts suggest performing aortic valve repair as the first line intervention in aortic valve regurgitation/insufficiency.[19]
The outcomes of aortic valve repair in aortic valve insufficiency rely on the size and quality of cusp available to repair. Patients with significant destruction of the aortic valve, calcifications, or mechanical damage due to rheumatic heart disease are less likely to benefit from aortic valve repair.[20] Aortic valve repair has shown positive outcomes in unicuspid, bicuspid, and quadricuspid aortic valve.[21][22]
Contraindications
A common contraindication to aortic valve repair surgery is a valvular apparatus that is not amenable to repair. Prior to planning on reparation, a careful evaluation of the valvular apparatus should be performed. Some of the factors that may preclude a repair procedure include excessively pliable or densely calcified, sclerotic, and stiff leaflets or valvular leaflet retraction.[23] A way to prevent such complications is not to offer surgical repair to patients with valvular apparatus that is not amenable to repair. A transesophageal echocardiogram to evaluate the entire valvular apparatus including an aortic annulus, aortic sinus, and sinotubular junction in addition to a thorough evaluation of aortic cusps should be performed prior to surgery to evaluate the entire heart. Valve repair should be performed in a center with appropriate physician expertise, and large volume centers should be the ones doing these procedures.
Equipment
Aortic valve-sparing surgery or valve repair is performed under general anesthesia. In addition to the surgical blades, saw, and operating knives, delicate instruments to perform aortic valve repair may be needed. Other instruments used include retractors, surgical sutures, and if plans for repairing aortic root or aorta, an aortic graft may also be needed as well.[24]
Personnel
Aortic valve repair should be performed in high volume centers. Anesthesiologists with experience in assisting with valve repair procedures are a crucial part of the team. In addition to the primary cardiothoracic surgeon performing the procedure, one or two junior assistant surgeons should be present at the time of surgery. Highly trained nursing staff should be part of the team, as well. Due to the paucity of the procedure performed in actual practice, well-trained surgical expertise is desirable when valve repair is planned.
Preparation
For planned valve repair procedures, patients should be fasting for eight or more hours prior to the procedure. Adequate pre-operative medical evaluation should be performed, including a comprehensive analysis of history, physical examination, and laboratory testing based on individual risk factors. Most patients usually undergo coronary artery evaluation prior to the valve repair procedure. However, this should be individualized since younger patients with congenital valve deformities may otherwise have no or few cardiac risk factors and hence not necessarily need a coronary artery disease evaluation.
Surgical time out should be performed by one of the team members, affirming the patient, the procedure to be performed, and all essential medications and allergies reviewed. After sedating the patient, the patient should be prepped and draped using sterile technique.
Technique
Surgical repair techniques are divided into two categories, including aortic root and annular remodeling, and cusp repair techniques. To better understand these techniques, it is important to know the three different types of aortic insufficiencies (AI). Type 1 corresponds to dilated aortic, causing cusps stretching and tethering and a central regurgitation jet. Type 2 is due to valve leaflet prolapse and presents as an eccentric jet. Type 3, which is least amenable to surgical repair, involves fibrosis and calcifications of the valve leading to cusp retraction. In most cases, all three of these co-exist to some degree.
Aortic Root and Annular Techniques: Type 1 aortic lesions in aortic sufficiency include pathology of any component of the functional annulus. These type 1 lesions can present alone or can accompany cusp pathology. In such cases, sub-commissural annuloplasty can improve cusp coaptation.[25] Type 1a aortic insufficiency has ascending aortic aneurysm with extension to STJ. Type 1a is related to the central jet of aortic insufficiency. The repair involves replacing the ascending aorta and closing the size of STJ with dacron graft sutures.
Valve Sparing Root Replacement (VSRR): After being first described in 1983, this technique has gradually gained popularity in patients with aortic root dilation and resultant aortic insufficiency.[26] It either consists of remodeling of the aortic root with annuloplasty or reimplantation of the aortic root with valve cusp assessment. In patients with tricuspid aortic valve and aortic insufficiency, causing a central regurgitation jet, this technique is being vastly utilized, particularly in high volume centers. The success of this technique has also been elaborated in bicuspid aortic valve patients with eccentric regurgitation jet.
Repair of Isolated Aortic Valve-related Aortic Insufficiency: In patients with Type 1 and Type 2 AI with preserved valvular apparatus, this technique is promising and has been practiced for a while. In type 3 AI, with less pliable valves, this technique might not be successful. Cusp prolapse, generations, and other deformities leading to AI can be effectively repaired. In addition, in patients with annulus and STJ abnormalities, a low threshold to repair of involved structures must be considered. The procedure may involve a circumferential subvalvular annuloplasty, and in patients with suspicion of supravalvular pathology, STJ annuloplasty or root replacement should be performed concomitantly.
Complications
The complications of aortic valve repair stem from inadequate repair itself in addition to other early procedural complications as well as delayed complications. As mentioned, aortic valve repair requires a certain degree of expertise to select appropriate patients for the appropriate procedures. However, even with that, certain complications from inadequate repair of the original valvular defect may persist.[27] They are listed as follows
Early Complications: Usually, due to a technical error.
- Low Coaptation: Inadequate valvular coaptation seen during intra-operative TEE or post-operative transesophageal echocardiogram should encourage the surgeon to continue the procedure and further plicate the cusps to prevent AI due to poor coaptation.
- Residual Prolapse: Any residual prolapse after completing the valve repair surgery either due to the surgical process or primary prolapse not related to surgery should be fixed prior to finishing the procedure.
- Cusp Perforation and Missed Fenestration: During manipulation of the valvular cusps, an iatrogenic perforation that happens should be immediately repaired. Fenestration in the valve, if large, should also be repaired prior to completing the procedure.
- Patch Dehiscence: In some cases, when a pericardial patch is used in the reimplantation of the aortic valve and aortic root, dehiscence of the patch may happen in the early phase of recovery necessitating a reoperation and fixation of the patch.
- Rupture of the Anterior Leaflet of Mitral Valve: Due to close proximity of the anterior leaflet of the mitral valve's base, an inadvertent perforation may lead to mitral regurgitation. This should be fixed in order to prevent hemodynamic compromise related to mitral insufficiency.
- Hematomas: Localized bleeding at the site of repair may lead to hematomas which, if small, usually reabsorb in few days.
- Complications Related to Subcommissural Annuloplasties (SCA): SCA can lead to disruption of the aortic valve apparatus at the subvalvular level and, in addition, fistulas formation and pseudoaneurysms. All these are severe complications that require fixation either during the index procedure or may require reoperation.
Delayed Complications: These are the complications that are encountered months to years after the valve replacement and are listed as follows:[28]
- Late-onset Persistent Aortic Insufficiency: Even with a successful procedure, some patients may end up having a small prolapse or mild mal-coaptation of the valve that may progress over the course of years to become thermodynamically significant. Similarly, gradual dilation of the aortic root can lead to progressive AI over the course of months or years.
- Endocarditis: Rarely endocarditis of the repaired valve has been reported in the literature.
- Bleeding: Though rare, bleeding in patients with aortic valve repair is noticed late after surgery. The mechanism is not clearly known.
- Thromboembolism: Late-onset thromboembolic events are rarely reported in these patients as well.
- Progressive Valve Fibrosis and Calcification: Attempts to repair type III AI may be initially successful but can eventually lead to further fibrosis and calcification of the valve.
Clinical Significance
Aortic valve repair procedures are being increasingly performed in patients with tricuspid aortic valves but also with bicuspid aortic valves with a slight difference in surgical techniques. In addition to providing the opportunity to repair the subvalvular and supravalvular apparatus including aortic root repair, they also provide the unique opportunity of repairing/remodeling the native valve. If performed with careful selection criteria and at a center with the proper expertise, outcomes comparable to that of valve replacement have been reported.[29]
Enhancing Healthcare Team Outcomes
The management of aortic regurgitation with aortic valve repair is challenging and complex. High volume centers with adequate expertise seem to a pre-requisite to gain procedural and long-term success. To derive good outcomes, the goals and objective of the aortic valve repair have to be defined prior to taking the patient to surgery. In some cases, a complication may necessitate further re-operation, thus requiring close coordination with the critical care teams for adequate patient recovery. As with any other complex procedure, the preoperative workup must be thorough, and the patient should be seen by a pulmonologist and cardiologist to optimize lung and cardiac function. Because of the potential risk of injury to the native valve, an intraoperative transesophageal echocardiogram (TEE), and a postoperative TEE monitoring is required.
Aortic valve repair has been reported to have good intermediate and long-term results. A perfect and standardized technique ensures the success of the procedure. Complications can occur immediately in the operating room, emphasizing the need for a continuous echocardiographic evaluation up to sternal closure. With the patient still on the operating table, it remains possible to go back to the bypass to immediately correct the problem.
In the postoperative period, the role of the nurse and pharmacist is critical. The nurses will assist the team by monitoring the patient for pain, bleeding, worsening of breathing, and a variety of common postoperative complications such as atelectasis, deep vein thrombosis, ileus, and pain. The pharmacist may be involved in preoperative and intraoperative medication reconciliation. The procedural success is dependent on close coordination between physicians, nurses, and pharmacists in in-patient and outpatient settings to detect both early and delayed complications and addressing them on time. Thus, further emphasizing the need for an interprofessional approach to the management of aortic insufficiency. The need for meticulous planning and discussion with other professionals involved in the management of the patient is highly recommended to lower the morbidity and improve outcomes.[29]
The repair of bicuspid aortic valve (BAV) repair deserves a special mention. In 1% to 2% of the patient population, a BAV is present. It may present as AI at a relatively young age.
(Click Image to Enlarge)
Valveguru [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)]
(Click Image to Enlarge)