Continuous Renal Replacement Therapy
- Article Author:
- Hollie Saunders
- Article Editor:
- Devang Sanghavi
- Updated:
- 7/31/2020 2:31:27 PM
- For CME on this topic:
- Continuous Renal Replacement Therapy CME
- PubMed Link:
- Continuous Renal Replacement Therapy
Introduction
Continuous renal replacement therapy is one of the renal replacement methods that include intermittent hemodialysis and peritoneal dialysis. It is intended to be applied for 24 hours or longer through continuous, slower dialysis. CRRT acts as renal support through blood purification to allow solute and fluid homeostasis. It requires appropriate vascular access, pumps to allow blood circulation, a permeable membrane, and varying solutions to allow fluid balance. There are different techniques of CRRT that are distinguished by their method of solute removal.
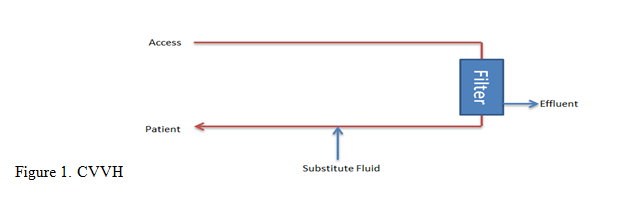
Continuous Venovenous Hemofiltration (CVVH)
This method utilizes convection via a transmembrane pressure gradient to filter solutes. It does not require dialysate fluid; instead, a substitute fluid is used to replace the filtered fluid.
Continuous Venovenous Hemodialysis (CVVHD)
This method utilizes diffusion via a transmembrane concentration gradient across the membrane. In this method, dialysate fluid is used. This is effective for solutes will small molecular weights such as potassium, urea, and creatinine.
Continuous Venovenous Hemodiafiltration (CVVHDF)
This combines both convection and diffusion methods of filtration.
The composition of the dialysate or the substitution fluids can be tailored to achieve the desired plasma composition. This is mainly applied in electrolyte derangements and in lactic acidosis, where a bicarbonate buffer may be used.
The preferred vascular access site is the right internal jugular vein due to a more direct pathway to the superior vena cava. An alternative to jugular access is femoral. Subclavian is less preferred as it may result in stenosis of the vessel and may cause future issues if the patient requires an arteriovenous (AV) fistula. End-stage renal disease (ESRD) patients who have AV fistulas should not use these if CRRT is needed. Using their established AV fistula increases the risk of needle dislodgment and bleeding.
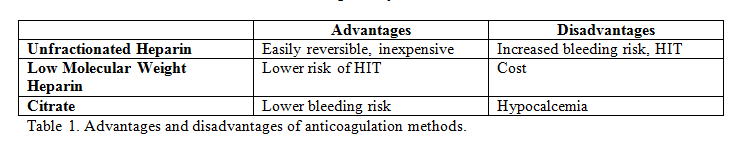
In CRRT, the use of an extracorporeal circuit results in a prothrombotic state. If membrane clotting occurs, it will result in a decrease in the surface area available for diffusion or convection. As such, an anticoagulant is required to prevent clotting. The anticoagulant used can either be systemic or regional. Both systemic and regional anticoagulation have advantages and disadvantages; these are detailed in table 1. The options for systemic anticoagulation include unfractionated heparin or low molecular weight heparin. If a patient has heparin-induced thrombocytopenia (HIT), alternatives such as argatroban or hirudin may be used for systemic anticoagulation. Regional anticoagulation is achieved using citrate. Citrate acts by chelating calcium and thus inhibiting the clotting cascade. If using this method, then serum ionized calcium levels should be monitored frequently. These patients will often require a calcium infusion with rate adjustments made based on the ionized calcium level. One option for if anticoagulation cannot be used is intermittent saline flushes. This is performed every 15 to 30 minutes and helps wash off the fibrin strands. However, filter half-lives are generally reduced.[1][2][3]. Table 1. Advantages and disadvantages of anticoagulation methods.
Indications
The indications for renal replacement in the acute setting often include those usually indicated for dialysis in acute renal failure (ARF), such as fluid overload, hyperkalemia, acidosis, and signs of uremia. The distinction comes in deciding on the method of renal replacement. The most common indication to choose continuous renal replacement therapy is in hemodynamically unstable patients. With a slower rate of fluid removal, CRRT theoretically causes less hypotension than intermittent hemodialysis (IHD). Its second advantage in these patients is that they often require large volume fluid administration, including medications and parenteral nutrition, and CRRT can prevent an overloaded state. Net fluid removal over 48 hours is greater than that in an IHD session. Another reason to choose CRRT is used for patients requiring dialysis, who have an acute brain injury. The use of CRRT in these patients can help prevent worsening cerebral edema. IHD may worsen cerebral edema in two ways, the rapid fluid removal will lower mean arterial pressure and cause compensatory cerebral vasodilation, and rapid removal of urea will cause fluid to shift from brain cells into the intracellular space. Inflammatory mediators such as interleukin 6 (IL-6), IL-8, IL-1, and tumor necrosis factor-alpha may also be removed via convection. This offers an advantage as CRRT is often used in sepsis syndromes.[1][2]
Contraindications
The main contraindication for CRRT is the need to have treatment outcomes reached more rapidly than the CRRT treatment can accomplish.
Complications
As with every procedure, CRRT has risks, and these should be communicated to the patient or family when considering initiation. Firstly, the risks associated with intravascular lines such as hemorrhage, infection, or thrombosis. The risks of the therapy itself include electrolyte disturbances, clearance of trace elements or antibiotics, hypothermia, and hypotension. Although hypotension should occur less commonly than in IHD, if the net ultrafiltration rate exceeds the rate of intravascular filling, then hypotension may occur. Monitoring of electrolytes and acid-base status should be done every 6-12 hours when starting CRRT. If remaining stable after the first 24 to 48 hours, the interval may be increased to 12-24 hours. The exception, as discussed above, is when using citrate as regional anticoagulation because this requires frequent monitoring of ionized calcium levels. The removal of medications during CRRT is variable, and so it is advised to check the dose of required medications when on CRRT. This practice is especially important when it comes to the administration of antibiotics, as the trough concentrations of these medications will determine their bacteriocidal or bacteriostatic effectiveness. Many patients who meet indications for CRRT will do so as a result of sepsis, meaning that appropriate antibiotic dosage is vital.[4][5] Finally, the risks associated with the extracorporeal circuit include hypersensitivity to the circuit, air embolization, as well as blood loss with filter or circuit changes.[1]
Clinical Significance
When to Initiate
There are many factors to consider when deciding whether to initiate CRRT. Two main factors are the severity of illness and the necessity of the procedure. The severity of the illness may be judged through the severity of the AKI and the observed trend. Further supporting factors would include the presence ofelectrolyte and acid-base disorders, evidence of fluid overload, and other significant organ dysfunction that requires renal support for promoting recovery. The necessity of the procedure may be judged through the assessment of the likelihood of the reversibility of the acute kidney injury (AKI), the presence of oliguria, and the nature and timing of the renal insult.
When to Discontinue
Every patient on CRRT should have daily monitoring for renal recovery. There are no standards for dialysis discontinuation. One way of monitoring renal recovery is through measuring urine output; increased urine output is an indicator of improving renal function. In addition to renal function, otherfactors such as fluid overload, ongoing hemodynamic instability, or continued need for nephrotoxic medication or large volumes may need to be considered before stopping CRRT.[6]
Enhancing Healthcare Team Outcomes
Continuous renal replacement therapy (CRRT) requires an interprofessional approach to be provided safely and efficiently. It requires the collaboration of specialties, including critical care, nephrology, and neurology, on essential elements such as when to initiate a mode of clearance, solute, and fluid removal targets, and anticoagulation strategies. Nursing is vital during CRRT as they have the most exposure to the vascular access site, the CRRT circuit itself, and the patient. Nursing staff and patient care technicians should be aware of complications so that intervention can be initiated early, and the physician team alerted promptly. In addition, pharmacists and nutritionists are vital to ensure proper medication doses and nutrition while on CRRT.
With this team approach and good education for each member, CRRT may be delivered as safely and effectively as possible.[7]
Listed below are the steps to provide optimal CRRT.
Nursing, Allied Health, and Interprofessional Team Interventions
- Collaboration between physician teams.
- Have the primary goal of CRRT defined.
- Ensure adequate access, machine and anticoagulation to maintain high functioning CRRT with minimal disruptions.
- Ensure appropriate nutrition support.
Nursing, Allied Health, and Interprofessional Team Monitoring
- Daily reassessment of CRRT prescription and response.
- Close attention to the appropriateness of medication dosing.
- Close lab and circuit monitoring for CRRT complications.
(Click Image to Enlarge)
(Click Image to Enlarge)