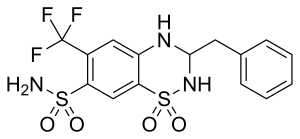
Bendroflumethiazide
 | |
 | |
| Names | |
|---|---|
IUPAC name
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | Oral |
| External links | |
| AHFS/Drugs.com | Consumer Drug Information |
| US NLM | Bendroflumethiazide |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 100% |
| Protein binding | 96% |
| Metabolism | extensive |
| Elimination half-life | 3-4 hours[2] |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ATC code | |
| Chemical and physical data | |
| Formula | C15H14F3N3O4S2 |
| Molar mass | 421.41 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Bendroflumethiazide, formerly bendrofluazide, trade name Aprinox,[3] is a medicine used to treat high blood pressure.[4] It is given as a single daily dose by mouth.[5]
Side effects include stomach ache, gout, low white count, pneumonitis and skin rash.[4] Bendroflumethiazide is a thiazide diuretic which works by inhibiting sodium reabsorption at the beginning of the distal convoluted tubule (DCT).[5] Water is lost as a result of more sodium reaching the collecting ducts. Bendroflumethiazide has a role in the treatment of mild heart failure although loop diuretics are better for reducing overload. The main use of bendroflumethiazide currently is in hypertension (part of the effect is due to vasodilation).
It was patented in 1958 and approved for medical use in 1960.[6] There is a combination version available with timolol.[4]
Side effects
Common adverse effects:[7]
- feeling dizzy due to orthostatic hypotension
- dry mouth or feeling thirsty
- nausea
- stomach ache
- fatigue
- diarrhea or constipation
- joint pain due to gout
Rare adverse effects:[8]
Alcohol
Bendroflumethiazide is known to have an adverse interaction with alcohol. It is advised that those using this diuretic should abstain from alcohol consumption during use, as it is possible to experience a sudden drop in blood pressure, especially if standing up (an effect known as orthostatic hypotension).
Other considerations
Bendroflumethiazide should not be used by pregnant women, or women who have just given birth. Due to the nature of the medication, it is possible for it to pass into the breast milk and consequently to the child. It is also known that bendroflumethiazide suppresses the production of breast milk. Pregnant or lactating women with hypertension may need to discuss with their prescriber as to which alternative treatment may be more suitable. Bendroflumethiazide may also impair the user's motor skills, therefore it is important to be aware of its effects and to take caution when operating machinery of driving.[7]
References
- ↑ BNF 45 March 2003
- ↑ Ed. Sean C. Sweetman (ed.). Martindale: The complete drug reference (33 ed.). Pharmaceutical Press.
- ↑ Xu, Q. Alan; Madden, Timothy L. (2011). Analytical Methods for Therapeutic Drug Monitoring and Toxicology. John Wiley & Sons. p. 58. ISBN 978-0-470-92279-8. Archived from the original on 2021-11-06. Retrieved 2021-11-06.
- 1 2 3 "2. Cardiovascular system". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. p. 181. ISBN 978-0-85711-413-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 Sam, Ramin; Pearce, David (2020). "15. Diuretic agents". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. pp. 272–273. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-11-06.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 456. ISBN 9783527607495. Archived from the original on 2021-11-01. Retrieved 2021-08-05.
- 1 2 "Bendroflumethiazide". NHS. Archived from the original on 2018-09-30. Retrieved 2018-09-29.
- ↑ "Bendroflumethiazide Side Effects". Drugs.com. Archived from the original on 2018-09-30. Retrieved 2018-09-29.