Bi-specific T-cell engager
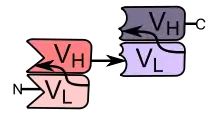
Bi-specific T-cell engagers (BiTEs) are a class of artificial bispecific monoclonal antibodies that are investigated for the use as anti-cancer drugs. They direct a host's immune system, more specifically the T cells' cytotoxic activity, against cancer cells. BiTE is a registered trademark of Micromet AG (fully owned subsidiary of Amgen Inc).[1]
BiTEs are fusion proteins consisting of two single-chain variable fragments (scFvs) of different antibodies, or amino acid sequences from four different genes, on a single peptide chain of about 55 kilodaltons. One of the scFvs binds to T cells via the CD3 receptor, and the other to a tumor cell via a tumor specific molecule.[2][3]
Mechanism of action

Like other bispecific antibodies, and unlike ordinary monoclonal antibodies, BiTEs form a link between T cells and tumor cells. This causes T cells to exert cytotoxic activity on tumor cells by producing proteins like perforin and granzymes, independently of the presence of MHC I or co-stimulatory molecules. These proteins enter tumor cells and initiate the cell's apoptosis.[2][4]
This action mimics physiological processes observed during T cell attacks against tumor cells.[4]
BiTEs in clinical assessment or with clinical approvals
Several BiTEs are currently in preclinical and clinical trials to assess their therapeutic efficacy and safety. [5]
Blinatumomab
Blinatumomab links T cells with CD19 receptors found the surface of B cells. The Food and Drug Administration (US) and the European Medicines Agency approved this therapy for adults with Philadelphia chromosome-negative relapsed or refractory acute lymphoblastic leukemia.[6]
Solitomab
Solitomab links T cells with the EpCAM antigen which is expressed by colon, gastric, prostate, ovarian, lung, and pancreatic cancers.[7][8]
Tebentafusp
After clinical trials, in Jan 2022 the US FDA approved tebentafusp (a BiTE targeting the gp100 peptide) for HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma.[9]
Further research
Utilizing the same technology, melanoma (with MCSP specific BiTEs) and acute myeloid leukemia (with CD33 specific BiTEs) can be targeted.[10] As of 2008 Research in this area is active.[10]
Another avenue for novel anti-cancer therapies is re-engineering some of the currently used conventional antibodies like trastuzumab (targeting HER2/neu), cetuximab and panitumumab (both targeting the EGF receptor), using the BiTE approach.[11]
As of 2009, BiTEs against CD66e and EphA2 are being developed as well.[12]
References
- ↑ "US Trademark registration no. 3,068,856, serial number 78/040,636". US Patent and Trademark Office.
- 1 2 Helwick, Caroline (1 June 2008). "Novel BiTE antibody mediates contact between T cells and cancer cells". Oncology NEWS International. 17 (6).
- ↑ Rüttinger, D.; Zugmaier, G.; Nagorsen, D.; Reinhardt, C.; Baeuerle, P. A. (2008). "BiTE-Antikörper: Durch Bispezifität T-Lymphozyten gegen Tumorzellen richten" [BiTE antibodies: Directing T lymphocytes against tumor cells by bispecifity]. Journal Onkologie (in German) (4).
- 1 2 "BiTE Antibody Platform". Micromet Inc.
- ↑ Voynov, V; Adam, PJ (2020). "Discovery Strategies to Maximize the Clinical Potential of T-Cell Engaging Antibodies for the Treatment of Solid Tumors". Antibodies. 9 (4): E65–E81. doi:10.3390/antib9040065. PMC 7709135. PMID 33217946. S2CID 227100306.
- ↑ Malard, Florent; Mohty, Mohamad (4 April 2020). "Acute lymphoblastic leukaemia". The Lancet. 395 (10230): 1146–1162. doi:10.1016/s0140-6736(19)33018-1. ISSN 0140-6736. PMID 32247396. S2CID 214779717.
- ↑ Amann, M.; d'Argouges, S.; Lorenczewski, G.; Brischwein, K.; Kischel, R.; Lutterbuese, R.; Mangold, S.; Rau, D.; Volkland, J.; Pflanz, S.; Raum, T.; Münz, M.; Kufer, P.; Schlereth, B.; Baeuerle, P. A.; Friedrich, M. (2009). "Antitumor Activity of an EpCAM/CD3-bispecific BiTE Antibody During Long-term Treatment of Mice in the Absence of T-cell Anergy and Sustained Cytokine Release". Journal of Immunotherapy. 32 (5): 452–464. doi:10.1097/CJI.0b013e3181a1c097. PMID 19609237. S2CID 25568468.
- ↑ Kebenko, Maxim; Goebeler, Marie-Elisabeth; Wolf, Martin; Hasenburg, Annette; Seggewiss-Bernhardt, Ruth; Ritter, Barbara; Rautenberg, Beate; Atanackovic, Djordje; Kratzer, Andrea; Rottman, James B.; Friedrich, Matthias (2018). "A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors". Oncoimmunology. 7 (8): e1450710. doi:10.1080/2162402X.2018.1450710. ISSN 2162-4011. PMC 6136859. PMID 30221040.
- ↑ FDA approves tebentafusp-tebn for unresectable or metastatic uveal melanoma Jan 2022
- 1 2 Kischel, R; et al. (2008). "Characterization in primates of MCSP- and CD33-specific human BiTE antibodies for treatment of Melanoma and AML" (PDF). Proc Am Assoc Cancer Res. 99. Abs 2404. Archived from the original (PDF) on 2011-07-19.
- ↑ Lutterbuese, R; et al. (2008). "Conversion of cetuximab, panitumumab, trastuzumab and omalizumab into T-cell-engaging BiTE antibodies creates novel drug candidates of high potency" (PDF). Proc Am Assoc Cancer Res. 99. Abs 2402. Archived from the original (PDF) on 2011-07-19.
- ↑ Baeuerle, PA; Reinhardt, C (2009). "Bispecific T-cell engaging antibodies for cancer therapy". Cancer Research. 69 (12): 4941–4. doi:10.1158/0008-5472.CAN-09-0547. PMID 19509221.
Further reading
- Kufer, P; Lutterbüse, R; Baeuerle, PA (2004). "A revival of bispecific antibodies". Trends in Biotechnology. 22 (5): 238–44. doi:10.1016/j.tibtech.2004.03.006. PMID 15109810.
