Cilazapril
 | |
| Names | |
|---|---|
| Preferred IUPAC name
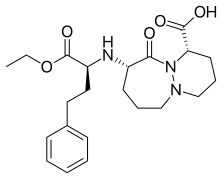
(1S,9S)-9-{[(2S)-1-Ethoxy-1-oxo-4-phenylbutan-2-yl]amino}-10-oxooctahydro-6H-pyridazino[1,2-a][1,2]diazepine-1-carboxylic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.168.764 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C22H31N3O5 |
| Molar mass | 417.506 g·mol−1 |
| log P | 2.212 |
| Acidity (pKa) | 2.285 |
| Basicity (pKb) | 11.712 |
| Pharmacology | |
| C09AA08 (WHO) | |
| Oral | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cilazapril is an angiotensin-converting enzyme inhibitor (ACE inhibitor) used for the treatment of hypertension and congestive heart failure.[1][2]
It was patented in 1982 and approved for medical use in 1990.[3]
Chemistry
Of the eight possible stereoisomers, only the all-(S)-form is medically viable.
Brand names
It is branded as Dynorm, Inhibace, Vascace and many other names in various countries. None of these are available in the United States as of May 2010.[4]
References
- ↑ Szucs, T. (1991). "Cilazapril. A review". Drugs. 41 Suppl 1: 18–24. doi:10.2165/00003495-199100411-00005. PMID 1712267.
- ↑ Jasek, W, ed. (2007). Austria-Codex (in German) (2007/2008 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-181-4.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 469. ISBN 9783527607495.
- ↑ "Cilazapril". Drugs.com. Retrieved 28 May 2010.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.