Congenital pulmonary airway malformation
| Congenital pulmonary airway malformation | |
|---|---|
| Other names: Congenital cystic adenomatoid malformation (CCAM)[1] | |
 | |
| Specialty | Medical genetics |
| Symptoms | None, increased work of breathing, bluish skin, swelling[2] |
| Complications | Pneumonia, pneumothorax, cancer[2] |
| Usual onset | Present at birth[2] |
| Types | Type 0 to 4[2] |
| Causes | Unknown[3] |
| Diagnostic method | Ultrasound before birth[2] |
| Differential diagnosis | Pulmonary sequestration, congenital lobar emphysema, pleuropulmonary blastoma[4] |
| Treatment | Conservative, surgery[2] |
| Frequency | 1 in 23,000 newborns[2] |
Congenital pulmonary airway malformation (CPAM) is a birth defect of the lung.[2] Symptoms can vary from none to increased work of breathing, bluish skin, and swelling.[2] Complications may include pneumonia, pneumothorax, or cancer.[2] About 5% of those affected are stillborn.[4]
The cause is generally unknown.[3][4] The underlying mechanism involves the formation of non functional lung tissue during early development.[2] There are five types: with type 1 being the most common and affecting one lobe of the lung; and type 2 being the next most common and occurring with multiple small cysts.[2] Other types are less common.[2] It is classified as a congenital thoracic malformation along with bronchogenic cysts and pulmonary sequestration.[2] Diagnosis is often by ultrasound before birth.[2]
Diagnosis before birth may allow the use of steroids or fetal surgery to improve outcomes.[2][3] If symptoms occur after birth or more than 20% of a lung is involved, surgery is generally recommended.[2] Otherwise either surgery or conservative management may be recommended.[2] Type 1 generally has a good outcome, while type 2 generally has a poor outcome.[2]
CPAM is rare, occurs in 1 in 10,000 to 35,000 newborns.[2] It is the most common birth defect affected the lungs.[2] Males are affected more often than females.[3] CPAM was first described in 1949 and divided into 5 types in 1977.[4][5]
Signs and symptoms
Three quarters of affected people are asymptomatic. About 25% develop cyanosis, pneumothorax, and show signs of increased breathing difficulty (tachypnoea and intercostal retractions).
At examination, they may show hyper-resonance at percussion, diminished vesicular murmur and an asymmetrical thorax.
Cause
The cause of CPAM is unknown.
Diagnosis
CPAMs are often identified during routine prenatal ultrasonography. Identifying characteristics on the sonogram include: an echogenic (bright) mass appearing in the chest of the fetus, displacement of the heart from its normal position, a flat or everted (pushed downward) diaphragm, or the absence of visible lung tissue.
CPAMs are classified into three different types based largely on their gross appearance. Type I has a large (>2 cm) multiloculated cysts. Type II has smaller uniform cysts. Type III is not grossly cystic, referred to as the "adenomatoid" type. Microscopically, the lesions are not true cysts, but communicate with the surrounding parenchyma. Some lesions have an abnormal connection to a blood vessel from an aorta and are referred to as "hybrid lesions."
Imaging
The earliest point at which a CPAM can be detected is by prenatal ultrasound. The classic description is of an echogenic lung mass that gradually disappears over subsequent ultrasounds. The disappearance is due to the malformation becoming filled with fluid over the course of the gestation, allowing the ultrasound waves to penetrate it more easily and rendering it invisible on sonographic imaging. When a CPAM is rapidly growing, either solid or with a dominant cyst, they have a higher incidence of developing venous outflow obstruction, cardiac failure and ultimately hydrops fetalis. If hydrops is not present, the fetus has a 95% chance of survival. When hydrops is present, risk of fetal demise is much greater without in utero surgery to correct the pathophysiology. The greatest period of growth is during the end of the second trimester, between 20–26 weeks.
A measure of mass volume divided by head circumference, termed cystic adenomatoid malformation volume ratio (CVR) has been developed to predict the risk of hydrops. The lung mass volume is determined using the formula (length × width × anteroposterior diameter ÷ 2), divided by head circumference. With a CVR greater than 1.6 being considered high risk. Fetuses with a CVR less than 1.6 and without a dominant cyst have less than a 3% risk of hydrops. After delivery, if the patient is symptomatic, resection is mandated. If the infant is asymptomatic, the need for resection is a subject of debate, though it is usually recommended. Development of recurrent infections, rhabdomyosarcoma, adenocarcinomas in situ within the lung malformation have been reported.[6]
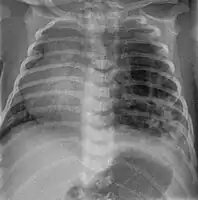 CPAM on chest radiograph in a newborn. Large cystic changes in the left lung, leading to a mediastinal shift to the right due to their mass effect.
CPAM on chest radiograph in a newborn. Large cystic changes in the left lung, leading to a mediastinal shift to the right due to their mass effect.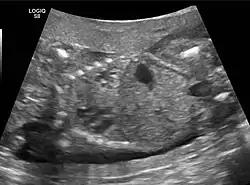 Congenital pulmonary airway malformation in a fetus, ultrasound at 19 weeks -sagittal. Stomach top right of image, heart displaced to bottom left of image (anatomically on the right side of fetus.)
Congenital pulmonary airway malformation in a fetus, ultrasound at 19 weeks -sagittal. Stomach top right of image, heart displaced to bottom left of image (anatomically on the right side of fetus.) Congenital pulmonary airway malformation in a fetus, ultrasound at 19 weeks - transverse. Stomach on left image; heart on right image: displaced to right by cystic mass
Congenital pulmonary airway malformation in a fetus, ultrasound at 19 weeks - transverse. Stomach on left image; heart on right image: displaced to right by cystic mass
Treatment
In most cases, a fetus with CPAM is closely monitored during pregnancy and the CPAM is removed via surgery after birth.[7] Most babies with a CPAM are born without complication and are monitored during the first few months. Many patients have surgery, typically before their first birthday, because of the risk of recurrent lung infections associated with CPAMs. Some pediatric surgeons can safely remove these lesions using very tiny incisions using minimally invasive surgical techniques (thoracoscopy). However, some CPAM patients live a full life without any complication or incident. It is hypothesized that there are thousands of people living with an undetected CPAM. Through ultrasound testing employed in recent years, many more patients are aware that they live with this condition. Rarely, long standing CPAMs have been reported to become cancerous.
Very large cystic masses might pose a danger during birth because of the airway compression. In this situation, a special surgical type of delivery called the EXIT procedure may be used.
In rare extreme cases, where fetus's heart is in danger, fetal surgery can be performed to remove the CPAM. If non-immune hydrops fetalis develop, there is a near universal mortality of the fetus without intervention. Fetal surgery can improve the chances of survival to 50-60%. Recently, several studies found that a single course of prenatal steroids (betamethasone) may increase survival in hydropic fetuses with microcystic CPAMs to 75-100%.[8][9] These studies indicate that large microcystic lesions may be treated prenatally without surgical intervention. Large macrocyst lesions may require in utero placement of a Harrison thoracoamniotic shunt.
References
- ↑ "Orphanet: Cystic adenomatoid malformation of the lung". www.orpha.net. Archived from the original on 28 August 2021. Retrieved 10 February 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Mehta, PA; Sharma, G (January 2020). "Congenital Pulmonary Airway Malformation". PMID 31869128.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 "Congenital Pulmonary Airway Malformation (CPAM) | Fetal Care Center". www.cincinnatichildrens.org. Archived from the original on 16 January 2021. Retrieved 11 February 2021.
- 1 2 3 4 Davenport, Mark; Coppi, Paolo De (2021). Paediatric Surgery. Oxford University Press. p. 144. ISBN 978-0-19-879869-9. Archived from the original on 2021-08-28. Retrieved 2021-02-11.
- ↑ Sellke, Frank; Nido, Pedro J. del; Swanson, Scott J. (2015). Sabiston and Spencer Surgery of the Chest E-Book. Elsevier Health Sciences. p. 166. ISBN 978-0-323-39377-5. Archived from the original on 2021-08-28. Retrieved 2021-02-11.
- ↑ Coley, editor-in-chief Brian D. (2013). Caffey's pediatric diagnostic imaging (Twelfth ed.). Philadelphia: Elsevier Saunders. ISBN 978-0323081764.
{{cite book}}:|first1=has generic name (help) - ↑ Ehrenberg-Buchner, S; Stapf, AM; Berman, DR; Drongowski, RA; Mychaliska, GB; Treadwell, MC; Kunisaki, SM (Nov 15, 2012). "Fetal lung lesions: can we start to breathe easier?". American Journal of Obstetrics and Gynecology. 208 (2): 151.e1–7. doi:10.1016/j.ajog.2012.11.012. PMID 23159697.
- ↑ Curran PF, Jelin EB, Rand L, Hirose S, Feldstein VA, Goldstein RB, Lee H (2010). "Prenatal steroids for microcystic congenital cystic adenomatoid malformations". J Pediatr Surg. 45 (1): 145–50. doi:10.1016/j.jpedsurg.2009.10.025. PMID 20105595. Archived from the original on 2021-08-28. Retrieved 2014-03-22.
- ↑ Peranteau WH, Wilson RD, Liechty KW, Johnson MP, Bebbington MW, Hedrick HL, Flake AW, Adzick NS (2007). "Effect of maternal betamethasone administration on prenatal congenital cystic adenomatoid malformation growth and fetal survival". Fetal Diagn Ther. 22 (5): 365–371. doi:10.1159/000103298. PMID 17556826. Archived from the original on 2019-05-18. Retrieved 2019-05-18.
External links
| Classification | |
|---|---|
| External resources |