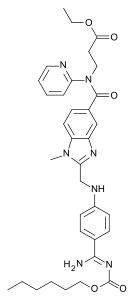
Dabigatran
 | |
| Names | |
|---|---|
| Trade names | Pradaxa, Pradax, Prazaxa, others |
| Other names | Dabigatran etexilate (USAN US) |
IUPAC name
| |
| Clinical data | |
| Pregnancy category | |
| Routes of use | By mouth |
| Defined daily dose | 300 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610024 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 3–7%[3] |
| Protein binding | 35%[3] |
| Elimination half-life | 12–17 hours[3] |
| Chemical and physical data | |
| Formula | C34H41N7O5 |
| Molar mass | 627.734 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Dabigatran, sold under the brand name Pradaxa among others, is an anticoagulant used to treat and prevent blood clots and to prevent stroke in people with atrial fibrillation.[3][4] Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots.[3] It is used as an alternative to warfarin and does not require monitoring by blood tests.[3] It is taken by mouth.[3]
Common side effects include bleeding and gastritis.[3] Other side effects may include bleeding around the spine and allergic reactions such as anaphylaxis.[3] In cases of severe bleeding, it can be reversed with the antidote, idarucizumab.[3] Use is not recommended during pregnancy or breastfeeding.[3] Compared to warfarin it has fewer interactions with other medications.[5] It is a direct thrombin inhibitor.[4]
Dabigatran was approved for medical use in the United States in 2010.[3] It is on the World Health Organization's List of Essential Medicines.[6] A month supply in the United Kingdom costs the NHS about GB£51 as of 2019.[4] In the United States the wholesale cost of this amount is about US$416.[7] In 2017, it was the 302nd most commonly prescribed medication in the United States, with more than one million prescriptions.[8]
Medical uses
Dabigatran is used to prevent strokes in those with atrial fibrillation not caused by heart valve issues, as well as deep vein thrombosis and pulmonary embolism in persons who have been treated for 5–10 days with parenteral anticoagulant (usually low molecular weight heparin), and to prevent deep vein thrombosis and pulmonary embolism in some circumstances.[9]
It appears to be as effective as warfarin in preventing non-hemorrhagic strokes and embolic events in those with atrial fibrillation not due to valve problems.[10]
Dosage
The defined daily dose is 300 mg by mouth.[2]
Contraindications
Dabigatran is contraindicated in patients who have active pathological bleeding, since dabigatran can increase bleeding risk and can also cause serious and potentially life-threatening bleeds.[11] Dabigatran is also contraindicated in patients who have a history of serious hypersensitivity reaction to dabigatran (e.g. anaphylaxis or anaphylactic shock).[11] The use of dabigatran should also be avoided in patients with mechanical prosthetic heart valves due to the increased risk of thromboembolic events (e.g. valve thrombosis, stroke, and myocardial infarction) and major bleeding associated with dabigatran in this population.[11][12][13]
Side effects
The most commonly reported side effect of dabigatran is gastrointestinal upset. When compared with people anticoagulated with warfarin, patients taking dabigatran had fewer life-threatening bleeds, fewer minor and major bleeds, including intracranial bleeds, but the rate of gastrointestinal bleeding was significantly higher. Dabigatran capsules contain tartaric acid, which lowers the gastric pH and is required for adequate absorption. The lower pH has previously been associated with dyspepsia; some hypothesize that this plays a role in the increased risk of gastrointestinal bleeding.[14]
A small but significantly increased risk of myocardial infarctions (heart attacks) has been noted when combining the safety outcome data from multiple trials.[15]
Reduced doses should be used in those with poor kidney function.[16]
Pharmacology
Mechanism of action
Dabigatran reversibly binds to the active site on the thrombin molecule, preventing thrombin-mediated activation of coagulation factors. Furthermore, dabigatran can inactivate thrombin even when thrombin is fibrin-bound; it reduces thrombin-mediated inhibition of fibrinolysis and, therefore, may enhance fibrinolysis.[17]
Pharmacokinetics
Dabigatran has a half-life of approximately 12–14 h and exerts a maximum anticoagulation effect within 2–3 h after ingestion. Fatty foods delay the absorption of dabigatran, although the bio-availability of the drug is unaffected.[18] One study showed that absorption may be moderately decreased if taken with a proton pump inhibitor.[19] Drug excretion through P-glycoprotein pumps is slowed in patients taking strong p-glycoprotein pump inhibitors such as quinidine, verapamil, and amiodarone, thus raising plasma levels of dabigatran.[20]
It is formulated as the prodrug dabigatran etexilate.[20]
History
Dabigatran (then compound BIBR 953) was discovered from a panel of chemicals with similar structure to benzamidine-based thrombin inhibitor α-NAPAP (N-alpha-(2-naphthylsulfonylglycyl)-4-amidinophenylalanine piperidide), which had been known since the 1980s as a powerful inhibitor of various serine proteases, specifically thrombin, but also trypsin. Addition of ethyl ester and hexyloxycarbonyl carbamide hydrophobic side chains led to the orally absorbed prodrug, BIBR 1048 (dabigatran etexilate).[21]
On 18 March 2008, the European Medicines Agency granted marketing authorisation for Pradaxa for the prevention of thromboembolic disease following hip or knee replacement surgery and for non-valvular atrial fibrillation.[22]
The National Health Service (NHS) in Britain authorised the use of dabigatran for use in preventing blood clots in hip and knee surgery patients. According to a BBC article in 2008, Dabigatran was expected to cost the NHS £4.20 per day, which was similar to several other anticoagulants.[23]
Initially, there was no specific way to reverse the anticoagulant effect of dabigatran in the event of a major bleeding event,[24] unlike for warfarin.[25] Since then, the dabigatran-specific antidote idarucizumab, a humanized monoclonal antibody for intravenous administration, was developed and received Food and Drug Administration (FDA) approval in 2015.[26]
Pradaxa received a Notice of Compliance (NOC) from Health Canada on 10 June 2008,[27] for the prevention of blood clots in patients who have undergone total hip or total knee replacement surgery. Approval for atrial fibrillation patients at risk of stroke came in October 2010.[28][29]
The U.S. Food and Drug Administration (FDA) approved Pradaxa on 19 October 2010, for prevention of stroke in patients with non-valvular atrial fibrillation.[30][31][32][33] The approval came after an advisory committee recommended the drug for approval on 20 September 2010[34] although caution is still urged by some outside experts.[35]
On 14 February 2011, the American College of Cardiology Foundation and the American Heart Association added dabigatran to their guidelines for management of non-valvular atrial fibrillation with a class I recommendation.[36]
In May 2014, the FDA reported the results of a large study comparing dabigatran with warfarin in 134,000 Medicare patients. The Agency concluded that dabigatran is associated with a lower risk of overall mortality, ischemic stroke, and bleeding in the brain than warfarin. Gastrointestinal bleeding was more common in those treated with dabigatran than in those treated with warfarin. The risk of heart attack was similar between the two drugs. The Agency reiterated its opinion that dabigatran's overall risk/benefit ratio is favorable.[37]
On 26 July 2014, the British Medical Journal (BMJ) published a series of investigations that accused Boehringer of withholding critical information about the need for monitoring to protect patients from severe bleeding, particularly in the elderly. Review of internal communications between Boehringer researchers and employees, the FDA and the EMA revealed that Boehringer researchers found evidence that serum levels of dabigatran vary widely. The BMJ investigation suggested that Boehringer had a financial motive to withhold this concern from regulatory health agencies because the data conflicted with their extensive marketing of dabigatran as an anticoagulant that does not require monitoring.[38][39]
Society and culture
Cost
A month supply in the United Kingdom costs the NHS about GB£51 as of 2019.[4]In the United States the wholesale cost of this amount is about US$416.[7] In 2017, it was the 302nd most commonly prescribed medication in the United States, with more than one million prescriptions.[8]
.svg.png.webp) DabigatranEtexilateMesylate costs (US)
DabigatranEtexilateMesylate costs (US).svg.png.webp) DabigatranEtexilateMesylate prescriptions (US)
DabigatranEtexilateMesylate prescriptions (US)
References
- 1 2 "Dabigatran (Pradaxa) Use During Pregnancy". Drugs.com. 27 December 2018. Archived from the original on 1 October 2020. Retrieved 16 May 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 8 July 2020. Retrieved 8 September 2020.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Dabigatran Etexilate Mesylate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 27 March 2019. Retrieved 27 March 2019.
- 1 2 3 4 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 135–137. ISBN 9780857113382.
- ↑ Kiser, Kathryn (2017). Oral Anticoagulation Therapy: Cases and Clinical Correlation. Springer. p. 11. ISBN 9783319546438. Archived from the original on 27 March 2019. Retrieved 30 July 2020.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- 1 2 "Dabigatran Etexilate Mesylate - Drug Usage Statistics". ClinCalc. Archived from the original on 28 February 2020. Retrieved 11 April 2020.
- ↑ https://www.drugs.com/pro/pradaxa.html Pradaxa - FDA prescribing information, side effects and uses at the Wayback Machine (archived 5 March 2020) Pradaxa
- ↑ Gómez-Outes A, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E (2013). "Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups". Thrombosis. 2013: 640723. doi:10.1155/2013/640723. PMC 3885278. PMID 24455237.
- 1 2 3 Pradaxa (dabigatran etexilate mesylate) Prescribing Information: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ba74e3cd-b06f-4145-b284-5fd6b84ff3c9#Section_5.4 DailyMed - PRADAXA- dabigatran etexilate mesylate capsule at the Wayback Machine (archived 28 June 2021), accessed 29 October 2014.
- ↑ "FDA Drug Safety Communication: Pradaxa (dabigatran etexilate mesylate) should not be used in patients with mechanical prosthetic heart valves". U.S. Food and Drug Administration (FDA). Archived from the original on 2 November 2014. Retrieved 29 October 2014.
- ↑ Eikelboom, JW; Connolly, SJ; Brueckmann, M; et al. (September 2013). "Dabigatran versus Warfarin in Patients with Mechanical Heart Valves". N Engl J Med. 369 (13): 1206–14. doi:10.1056/NEJMoa1300615. PMID 23991661.
- ↑ ML Blommel (2011). "Dabigatran etexilate: A novel oral direct thrombin inhibitor". Am J Health Syst Pharm. 68 (16): 1506–19. doi:10.2146/ajhp100348. PMID 21817082.
- ↑ Uchino K; Hernandez AV (2012). "Dabigatran associated with higher risk of acute coronary events - meta-analysis of noninferiority randomized controlled trials". Arch. Intern. Med. 172 (5): 397–402. doi:10.1001/archinternmed.2011.1666. PMID 22231617. Archived from the original on 23 April 2012.
- ↑ "18/12/2014 Pradaxa -EMEA/H/C/000829 -II/0073".
{{cite web}}: CS1 maint: url-status (link) - ↑ Kallmes, D. F.; Comin, J. (1 March 2012). "Dabigatran (Pradaxa)". American Journal of Neuroradiology. 33 (3): 426–428. doi:10.3174/ajnr.A3000. ISSN 0195-6108. PMID 22345499. Archived from the original on 31 July 2020. Retrieved 30 July 2020.
- ↑ Pradaxa Full Prescribing Information Wayback Machine at the Wayback Machine (archived 2015-08-10). Boehringer Ingelheim. October 2010.
- ↑ Stangier J; Eriksson BI; Dahl OE (May 2005). "Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement". J Clin Pharmacol. 45 (5): 555–63. doi:10.1177/0091270005274550. PMID 15831779.
- 1 2 "Pradaxa Summary of Product Characteristics" (2018) Wayback Machine at the Wayback Machine (archived 2019-07-05). European Medicines Agency.
- ↑ Hauel NH; Nar H; Priepke H; Ries U; Stassen JM; Wienen W (April 2002). "Structure-based design of novel potent nonpeptide thrombin inhibitors". J Med Chem. 45 (9): 1757–66. doi:10.1021/jm0109513. PMID 11960487.
- Lay summary in: https://montrealgazette.com/health/anti+clotting+drug+heralded/3737627/story.html.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)
- Lay summary in: https://montrealgazette.com/health/anti+clotting+drug+heralded/3737627/story.html.
- ↑ "Pradaxa EPAR". European Medicines Agency. Archived from the original on 2 August 2012. Retrieved 30 January 2011.
- ↑ "Clot drug 'could save thousands'". BBC News Online. 20 April 2008. Archived from the original on 15 January 2009. Retrieved 21 April 2008.
- ↑ van Ryn J; Stangier J; Haertter S; Liesenfeld KH; Wienen W; Feuring M; Clemens A (June 2010). "Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity". Thrombosis and Haemostasis. 103 (6): 1116–27. doi:10.1160/TH09-11-0758. PMID 20352166.
Although there is no specific antidote to antagonise the anticoagulant effect of dabigatran, due to its short duration of effect drug discontinuation is usually sufficient to reverse any excessive anticoagulant activity.
- ↑ Hanley, JP (November 2004). "Warfarin reversal". Journal of Clinical Pathology. 57 (11): 1132–9. doi:10.1136/jcp.2003.008904. PMC 1770479. PMID 15509671.
- ↑ Syed, Yahiya Y. (7 July 2016). "Idarucizumab: A Review as a Reversal Agent for Dabigatran". American Journal of Cardiovascular Drugs. 16 (4): 297–304. doi:10.1007/s40256-016-0181-4. PMID 27388764.
- ↑ "Summary Basis of Decision (SBD): Pradax" Wayback Machine at the Wayback Machine (archived 14 July 2016) Health Canada. 2008-11-06.
- ↑ Kirkey, Sharon (29 October 2010). "Approval of new drug heralds 'momentous' advance in stroke prevention". Montreal Gazette. Archived from the original on 16 June 2011. Retrieved 29 October 2010.
- ↑ "Pradax (Dabigatran Etexilate) Gains Approval In Canada For Stroke Prevention In Atrial Fibrillation" PRADAX™ (Dabigatran Etexilate) Gains Approval In Canada For Stroke Prevention In Atrial Fibrillation at the Wayback Machine (archived 24 April 2011) Medical News Today. 28 October 2010.
- ↑ Connolly, SJ; Ezekowitz, MD; Yusuf, S; et al. (September 2009). "Dabigatran versus warfarin in patients with atrial fibrillation". N Engl J Med. 361 (12): 1139–51. doi:10.1056/NEJMoa0905561. PMID 19717844. Archived from the original on 28 August 2021. Retrieved 30 July 2020.
- ↑ Turpie AG (January 2008). "New oral anticoagulants in atrial fibrillation". Eur Heart J. 29 (2): 155–65. doi:10.1093/eurheartj/ehm575. PMID 18096568.
- ↑ "Boehringer wins first US OK in blood-thinner race". Reuters. 19 October 2010. Archived from the original on 10 November 2013. Retrieved 20 October 2010.
- ↑ "FDA approves Pradaxa to prevent stroke in people with atrial fibrillation" (Press release). U.S. Food and Drug Administration (FDA). 19 October 2010. Archived from the original on 18 January 2017. Retrieved 30 July 2020.
- ↑ Shirley S. Wang (20 September 2010). "New Blood-Thinner Recommended by FDA Panel". The Wall Street Journal. Archived from the original on 24 April 2016. Retrieved 20 October 2010.
- ↑ Merli G; Spyropoulos AC; Caprini JA (August 2009). "Use of emerging oral anticoagulants in clinical practice: translating results from clinical trials to orthopedic and general surgical patient populations". Ann Surg. 250 (2): 219–28. doi:10.1097/SLA.0b013e3181ae6dbe. PMID 19638915.
- ↑ Wann LS; Curtis AB; Ellenbogen KA; Estes NA; Ezekowitz MD; Jackman WM; January CT; Lowe JE; Page RL; Slotwiner DJ; Stevenson WG; Tracy CM; Jacobs AK; Rydén; Cannom; Crijns; Curtis; Ellenbogen; Halperin; Kay; Le Heuzey; Lowe; Olsson; Prystowsky; Tamargo; Wann; Jacobs; Anderson; Albert; et al. (March 2011). "2011 ACCF/AHA/HRS Focused Update on the Management of Patients With Atrial Fibrillation (Update on Dabigatran): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". Circulation. 123 (10): 1144–50. doi:10.1161/CIR.0b013e31820f14c0. PMID 21321155.
- ↑ "FDA Drug Safety Communication: FDA study of Medicare patients finds risks lower for stroke and death but higher for gastrointestinal bleeding with Pradaxa (dabigatran) compared to warfarin". Archived from the original on 23 April 2019.
- ↑ Cohen, D (July 2014). "Dabigatran: how the drug company withheld important analyses". BMJ. 349: g4670. doi:10.1136/bmj.g4670. PMID 25055829.
- ↑ Moore TJ; Cohen MR; Mattison DR (July 2014). "Dabigatran, bleeding, and the regulators". BMJ. 349: g4517. doi:10.1136/bmj.g4517. PMID 25056265.
External links
- "Dabigatran". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 17 July 2020. Retrieved 30 July 2020.
- "Dabigatran etexilate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 5 July 2020. Retrieved 30 July 2020.
| Identifiers: |
|---|