EMPA (drug)
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
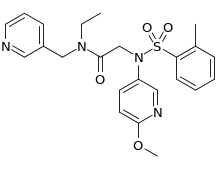
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.233.393 |
| Chemical and physical data | |
| Formula | C23H26N4O4S |
| Molar mass | 454.55 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
EMPA is a selective antagonist of the OX2 receptor, with 900-fold selectivity in binding for OX2 over OX1.[1][2]
See also
References
- ↑ Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F (September 2009). "Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists". Molecular Pharmacology. 76 (3): 618–31. doi:10.1124/mol.109.055152. PMID 19542319. S2CID 5702143.
- ↑ Malherbe P, Borroni E, Gobbi L, Knust H, Nettekoven M, Pinard E, et al. (April 2009). "Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX(2) receptor". British Journal of Pharmacology. 156 (8): 1326–41. doi:10.1111/j.1476-5381.2009.00127.x. PMC 2697736. PMID 19751316.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.