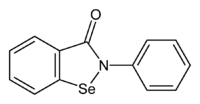
Ebselen
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Phenyl-1,2-benzoselenazol-3(2H)-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.132.190 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C13H9NOSe |
| Molar mass | 274.17666 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ebselen (also called PZ 51, DR3305, and SPI-1005), is a synthetic organoselenium drug molecule with anti-inflammatory, anti-oxidant and cytoprotective activity. It acts as a mimic of glutathione peroxidase and can also react with peroxynitrite.[1] It is being investigated as a possible treatment for reperfusion injury and stroke,[2][3] hearing loss and tinnitus,[4] and bipolar disorder.[5][6]
Additionally, ebselen may be effective against Clostridioides difficile infections[7] and has been shown to have antifungal activity against Aspergillus fumigatus.[8]
Ebselen is a potent scavenger of hydrogen peroxide as well as hydroperoxides including membrane bound phospholipid and cholesterylester hydroperoxides. Several ebselen analogs have been shown to scavenge hydrogen peroxide in the presence of thiols.[9]
Possible anti-COVID-19 activity
Preliminary studies demonstrate that Ebselen exhibits promising inhibitory activity against COVID-19 in cell-based assays.[10][11][12] The effect was attributed to irreversible inhibition of the main protease via a covalent bond formation with the thiol group of the active center's cysteine (Cys-145).[10]
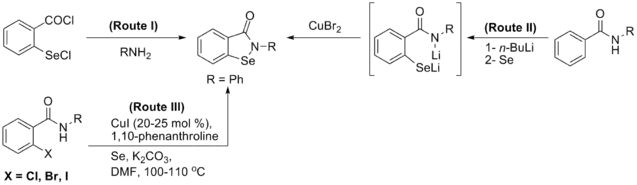
Synthesis
Generally, synthesis of the characteristic scaffold of ebselen, the benzoisoselenazolone ring system, can be achieved either through reaction of primary amines (RNH2) with 2-(chloroseleno)benzoyl chloride (Route I),[13] by ortho-lithiation of benzanilides followed by oxidative cyclization (Route II) mediated by cupric bromide (CuBr2),[14] or through the efficient Cu-catalyzed selenation / heterocyclization of o-halobenzamides, a methodology developed by Kumar et al.[15] (Route III).
References
- ↑ Schewe T (October 1995). "Molecular actions of ebselen--an antiinflammatory antioxidant". General Pharmacology. 26 (6): 1153–69. doi:10.1016/0306-3623(95)00003-J. PMID 7590103.
- ↑ Parnham M, Sies H (March 2000). "Ebselen: prospective therapy for cerebral ischaemia". Expert Opinion on Investigational Drugs. 9 (3): 607–19. doi:10.1517/13543784.9.3.607. PMID 11060699.
- ↑ Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H (January 1998). "Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group". Stroke. 29 (1): 12–7. doi:10.1161/01.STR.29.1.12. PMID 9445321.
- ↑ Kil J, Pierce C, Tran H, Gu R, Lynch ED (April 2007). "Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase". Hearing Research. 226 (1–2): 44–51. doi:10.1016/j.heares.2006.08.006. PMID 17030476.
- ↑ Singh N, Halliday AC, Thomas JM, Kuznetsova OV, Baldwin R, Woon EC, et al. (2013). "A safe lithium mimetic for bipolar disorder". Nature Communications. 4: 1332. Bibcode:2013NatCo...4.1332S. doi:10.1038/ncomms2320. PMC 3605789. PMID 23299882.
- ↑ "New drug for bipolar disorder may offer fewer side effects". University of Oxford Latest News. 2013-01-08. Retrieved 12 January 2013.
- ↑ "Drug disarms deadly C. difficile bacteria without destroying healthy gut flora". Medical Express.
- ↑ Marshall AC, Kidd SE, Lamont-Friedrich SJ, Arentz G, Hoffmann P, Coad BR, Bruning JB (March 2019). "Structure, Mechanism, and Inhibition of Aspergillus fumigatus Thioredoxin Reductase". Antimicrobial Agents and Chemotherapy. 63 (3): e02281-18. doi:10.1128/AAC.02281-18. PMC 6395915. PMID 30642940.
- ↑ Satheeshkumar K, Mugesh G (April 2011). "Synthesis and antioxidant activity of peptide-based ebselen analogues". Chemistry. 17 (17): 4849–57. doi:10.1002/chem.201003417. PMID 21400619.
- 1 2 Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. (June 2020). "Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors". Nature. 582 (7811): 289–293. doi:10.1038/s41586-020-2223-y. PMID 32272481.
- ↑ Weglarz-Tomczak E, Tomczak JM, Talma M, Burda-Grabowska M, Giurg M, Brul S (February 2021). "Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2". Scientific Reports. 11 (1): 3640. doi:10.1038/s41598-021-83229-6. PMC 7878891. PMID 33574416.
- ↑ Xiang R, Yu Z, Wang Y, Wang L, Huo S, Li Y, et al. (July 2021). "Recent advances in developing small-molecule inhibitors against SARS-CoV-2". Acta Pharmaceutica Sinica. B. doi:10.1016/j.apsb.2021.06.016. PMC 8260826. PMID 34249607.
- ↑ Kamigata N, Iizuka H, Izuoka A, Kobayashi M (July 1986). "Photochemical Reaction of 2-Aryl-1, 2-benzisoselenazol-3 (2 H)-ones". Bulletin of the Chemical Society of Japan. 59 (7): 2179–83. doi:10.1246/bcsj.59.217.
- ↑ Engman L, Hallberg A (1989-06-01). "Expedient synthesis of ebselen and related compounds". The Journal of Organic Chemistry. 54 (12): 2964–2966. doi:10.1021/jo00273a035. ISSN 0022-3263.
- ↑ Balkrishna SJ, Bhakuni BS, Chopra D, Kumar S (December 2010). "Cu-catalyzed efficient synthetic methodology for ebselen and related Se-N heterocycles". Organic Letters. 12 (23): 5394–7. doi:10.1021/ol102027j. PMID 21053969.