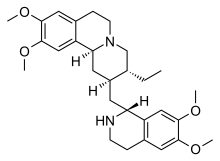
Emetine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.903 |
| Chemical and physical data | |
| Formula | C29H40N2O4 |
| Molar mass | 480.649 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Emetine is a drug used as both an anti-protozoal and to induce vomiting. It is produced from the ipecac root. It takes its name from its emetic properties.[1]
Early preparations
Mechanism of action of emetine was studied by François Magendie during the nineteenth century.
Early use of emetine was in the form of oral administration of the extract of ipecac root, or ipecacuanha. This extract was originally thought to contain only one alkaloid, emetine, but was found to contain several, including cephaeline, psychotrine and others. Although this therapy was reportedly successful, the extract caused vomiting in many patients, which reduced its utility. In some cases, it was given with opioids to reduce nausea. Other approaches to reduce nausea involved coated tablets, allowing the drug to be released after digestion in the stomach.[2]
Use as anti-amoebic
The identification of emetine as a more potent agent improved the treatment of amoebiasis. While use of emetine still caused nausea, it was more effective than the crude extract of ipecac root. Additionally, emetine could be administered hypodermically which still produced nausea, but not to the degree experienced in oral administration.
Although it is a potent antiprotozoal, the drug also can interfere with muscle contractions, leading to cardiac failure in some cases. Because of this, in some uses it is required to be administered in a hospital so that adverse events can be addressed.
Dehydroemetine
Dehydroemetine is a synthetically produced antiprotozoal agent similar to emetine in its anti-amoebic properties and structure (they differ only in a double bond next to the ethyl group), but it produces fewer side effects.
Cephaeline
Cephaeline is a desmethyl analog of emetine also found in ipecac root.
Use in blocking protein synthesis
Emetine dihydrochloro hydrate is used in the laboratory to block protein synthesis in eukaryotic cells. It does this by binding to the 40S subunit of the ribosome.[3] This can thus be used in the study of protein degradation in cells. Mutants resistant to emetine are altered in the 40S ribosomal subunit (S14 protein),[4][5] and they exhibit cross-resistance to cryptopleurine, tylocrebrine, cephaeline and tubulosine, but not other inhibitors of protein synthesis.[6] The compounds to which these mutants exhibit cross-resistance have been shown to share common structural determinants with emetine that are responsible for their biological activities.[7]
Biosynthesis
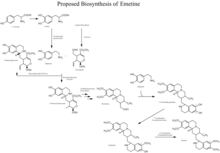
The biosynthesis of cephaeline and emetine come from two main biosynthesis pathways: the biosynthesis of dopamine from L-tyrosine and the biosynthesis of secologanin from geranyl diphosphate. Biosynthesis begins from the reaction between dopamine and secologanin forming N-deacetylisoipecoside (S-form) and N-deacetylipecoside (R-form). The S-form then goes through a Pictet-Spengler type reaction followed by a series of O-methylations and the removal of glucose, with O-methyltransferases and a glycosidase, to form proemetine. Proemetine then reacts with another dopamine molecule to form 7'-O-demethylcephaeline. The final products are then produced with a 7'-O-methylation to make cephaeline and a 6'-O-methylation successively to make emetine.[8][9]
Side effects
Heavy or overusage of emetine can carry the risk of developing proximal myopathy and/or cardiomyopathy.[10]
Research
A 2018 study [11] at Princeton University and Thomas Jefferson University has demonstrated that emetine blocks the dissemination of rabies virus inside nerve cells, but the exact mechanism is still under investigation. Emetine had no effect on the transport of endosomes devoid of the rabies virus. (Rabies resides in nerve endosomes). But endosomes carrying the virus were either completely immobilized, or were only able to move short distances at slower-than-normal speeds.[12]
In 2016, a study[13] found that low doses of emetine inhibited cytomegalovirus replication and was synergistic with ganciclovir.
References
- ↑ "NCATS Inxight: Drugs". drugs.ncats.io. Retrieved 2020-01-22.
- ↑ Cushny AR (1918). A Textbook of pharmacology and therapeutics, or the action of drugs in health and disease. Lea and Febiger, New York. pp. 438–442.
emetine.
- ↑ Jiménez A, Carrasco L, Vázquez D (October 1977). "Enzymic and nonenzymic translocation by yeast polysomes. Site of action of a number of inhibitors". Biochemistry. 16 (21): 4727–30. doi:10.1021/bi00640a030. PMID 334249.
- ↑ Gupta RS, Siminovitch L (January 1977). "The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit". Cell. 10 (1): 61–6. doi:10.1016/0092-8674(77)90140-4. PMID 837444.
- ↑ Rhoads DD, Roufa DJ (July 1985). "Emetine resistance of Chinese hamster cells: structures of wild-type and mutant ribosomal protein S14 mRNAs". Molecular and Cellular Biology. 5 (7): 1655–9. doi:10.1128/mcb.5.7.1655. PMC 367284. PMID 3839563.
- ↑ Gupta RS, Siminovitch L (July 1977). "Mutants of CHO cells resistant to the protein synthesis inhibitors, cryptopleurine and tylocrebrine: genetic and biochemical evidence for common site of action of emetine, cryptopleurine, tylocrebine, and tubulosine". Biochemistry. 16 (14): 3209–14. doi:10.1021/bi00633a026. PMID 560858.
- ↑ Gupta RS, Krepinsky JJ, Siminovitch L (July 1980). "Structural determinants responsible for the biological activity of (-)-emetine, (-)-cryptopleurine, and (-)-tylocrebrine: structure-activity relationship among related compounds". Molecular Pharmacology. 18 (1): 136–43. PMID 7412757.
- ↑ Cheong BE, Takemura T, Yoshimatsu K, Sato F (2011). "Molecular cloning of an O-methyltransferase from adventitious roots of Carapichea ipecacuanha" (PDF). Bioscience, Biotechnology, and Biochemistry. 75 (1): 107–13. doi:10.1271/bbb.100605. PMID 21228475.
- ↑ Nomura T, Kutchan TM (March 2010). "Three new O-methyltransferases are sufficient for all O-methylation reactions of ipecac alkaloid biosynthesis in root culture of Psychotria ipecacuanha". The Journal of Biological Chemistry. 285 (10): 7722–38. doi:10.1074/jbc.M109.086157. PMC 2844217. PMID 20061395.
- ↑ "NCATS Inxight: Drugs". drugs.ncats.io. Retrieved 2020-01-22.
- ↑ MacGibeny MA, Koyuncu OO, Wirblich C, Schnell MJ, Enquist LW (July 2018). "Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons". PLOS Pathogens. 14 (7): e1007188. doi:10.1371/journal.ppat.1007188. PMC 6070286. PMID 30028873.
- ↑ "How rabies virus moves through nerve cells, and how it might be stopped".
- ↑ Mukhopadhyay R, Roy S, Venkatadri R, Su YP, Ye W, Barnaeva E, et al. (June 2016). "Efficacy and Mechanism of Action of Low Dose Emetine against Human Cytomegalovirus". PLOS Pathogens. 12 (6): e1005717. doi:10.1371/journal.ppat.1005717. PMC 4919066. PMID 27336364.