Levamisole
 | |
 | |
| Names | |
|---|---|
| Trade names | Ergamisol |
IUPAC name
| |
| Clinical data | |
| Drug class | Antihelmintic[1] |
| Main uses | Ascariasis, hookworm infections[2] |
| Side effects | Abdominal pain, vomiting, headache, dizziness[2] |
| Routes of use | By mouth[3] |
| Defined daily dose | 0.15 gram[4] |
| External links | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| US NLM | Levamisole |
| MedlinePlus | a697011 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Liver |
| Elimination half-life | 3–4 hours |
| Excretion | Urine (70%) |
| Chemical and physical data | |
| Formula | C11H12N2S |
| Molar mass | 204.29 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.31 g/cm3 |
| Melting point | 60 °C (140 °F) |
SMILES
| |
InChI
| |
Levamisole, sold under the brand name Ergamisol among others, is a medication used to treat parasitic worm infections.[5] Specifically it is used for ascariasis and hookworm infections.[2] It is taken by mouth.[2]
Side effects may include abdominal pain, vomiting, headache, and dizziness.[2] Use is not recommended during breastfeeding or the third trimester of pregnancy.[2] Serious side effects may include an increased risk of infection.[1] It belongs to the antihelmintic class of medications.[1]
Levamisole was discovered in 1966.[6] It is on the World Health Organization's List of Essential Medicines.[7] The wholesale cost in the developing world is about US$0.18 to US$0.33 for a course of treatment.[8] It is not commercially available in the United States.[1] Levamisole is also used as a dewormer for livestock.[9]
Medical uses
Worms
Levamisole was originally used as an anthelmintic to treat worm infestations in both humans and animals. Levamisole works as a nicotinic acetylcholine receptor agonist that causes continued stimulation of the parasitic worm muscles, leading to paralysis. In countries that still permit the use of levamisole, the recommended dose for anthelmintic therapy is a single dose, with a repeated dose 7 days later if needed for a severe hookworm infection.[10] Most current commercial preparations are intended for veterinary use as a dewormer in cattle, pigs, and sheep. However, levamisole has also recently gained prominence among aquarists as an effective treatment for Camallanus roundworm infestations in freshwater tropical fish.[11]
Cancer
After being pulled from the market in the U.S. and Canada in 1999 and 2003, respectively, levamisole has been tested in combination with fluorouracil to treat colon cancer. Evidence from clinical trials support its addition to fluorouracil therapy to benefit patients with colon cancer. In some of the leukemic cell line studies, both levamisole and tetramisole showed similar effect.[12]
Other
Levamisole has been used to treat a variety of dermatologic conditions, including skin infections, leprosy, warts, lichen planus, and aphthous ulcers.[13]
An interesting adverse side effect these reviewers reported in passing was "neurologic excitement". Later papers, from the Janssen group and others, indicate levamisole and its enantiomer, dexamisole, have some mood-elevating or antidepressant properties, although this was never a marketed use of the drug.[14][15]
Dosage
The defined daily dose is 0.15 gram (by mouth)[4]
Side effects
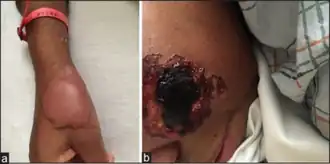
One of the more serious side effects of levamisole is agranulocytosis, or the depletion of the white blood cells. In particular, neutrophils appear to be affected the most. This occurs in 0.08–5% of the studied populations.[16] There have also been reports of levamisole induced necrosis syndrome in which erythematous painful papules can appear almost anywhere on skin.
It has been used as an adulterant in cocaine resulting in serious side effects.[17][18][19]
Metabolism
Levamisole is readily absorbed from the gastrointestinal tract and metabolized in the liver. Its time to peak plasma concentration is 1.5–2 hours. The plasma elimination half-life is fairly quick at 3–4 hours which can contribute to not detecting Levamisole intoxication. The metabolite half-life is 16 hours. Levamisole's excretion is primarily through the kidneys, with about 70% being excreted over 3 days. Only about 5% is excreted as unchanged levamisole.[20][21]
Drug testing of racehorse urine has led to the revelation that among levamisole equine metabolites are both pemoline and aminorex, stimulants that are forbidden by racing authorities.[22][23][24] Further testing confirmed aminorex in human and canine urine, meaning that both humans and dogs also metabolize levamisole into aminorex.,[25] though it is unclear whether plasma aminorex is present at any appreciable level. Blood samples following oral administration of levamisole out to 172 hr post-dose did not demonstrate any plasma aminorex levels above that of the limit of quantification (LoQ). Additionally, in cocaine-positive plasma samples, of which 42% contained levamisole, aminorex was never reported at concentrations higher than LoQ.[26]
Detection in body fluids
Levamisole may be quantified in blood, plasma, or urine as a diagnostic tool in clinical poisoning situations or to aid in the medicolegal investigation of suspicious deaths involving adulterated street drugs. About 3% of an oral dose is eliminated unchanged in the 24-hour urine of humans. A post mortem blood levamisole concentration of 2.2 mg/L was present in a woman who died of a cocaine overdose.[27][28]
Illicit use
Levamisole has increasingly been used as a cutting agent in cocaine sold around the globe with the highest incidence being in the USA. In 2008–2009, levamisole was found in 69% of cocaine samples seized by the Drug Enforcement Administration (DEA).[17] By April 2011, the DEA reported the adulterant was found in 82% of seizures.[29]
Levamisole adds bulk and weight to powdered cocaine (whereas other adulterants produce smaller "rocks" of cocaine) and makes the drug appear purer.[30] In a series of investigative articles for The Stranger, Brendan Kiley details other rationales for levamisole's rise as an adulterant: possible stimulant effects, a similar appearance to cocaine, and an ability to pass street purity tests.[31]
Levamisole suppresses the production of white blood cells, resulting in neutropenia and agranulocytosis. With the increasing use of levamisole as an adulterant, a number of these complications have been reported among cocaine users.[17][32][33] Levamisole has also been linked to a risk of vasculitis,[34] and two cases of vasculitic skin necrosis have been reported in users of cocaine adulterated with levamisole.[35]
Levamisole-tainted cocaine was linked to several high-profile deaths. Toxicology reports showed levamisole, along with cocaine, was present in DJ AM's body at the time of his death.[36] Andrew Koppel, son of newsman Ted Koppel, was also found with levamisole in his body after his death was ruled a drug overdose.[37] In 2014 it was suspected to be involved in the death of a Sydney teenager.[38]
In response to the dangers, The Stranger, People's Harm-Reduction Alliance, and DanceSafe began producing tests to identify levamisole's presence in cocaine. The kits include a survey postcard, and one revealed its presence in a 1/4-kg block of cocaine, indicating both users and dealers were using the kits.[39]
Chemistry
The original synthesis at Janssen Pharmaceutica resulted in the preparation of a racemic mixture of two enantiomers, whose hydrochloride salt was reported to have a melting point of 264–265 °C; the free base of the racemate has a melting point of 87–89 °C. The racemic mixture is referred to as "tetramisole" - levamisole refers only to the levorotatory enantiomer of tetramisole.
Toxicity
Laboratory use
Levamisole reversibly and noncompetitively inhibits most isoforms of alkaline phosphatase (e.g., human liver, bone, kidney, and spleen) except the intestinal and placental isoform.[41] It is thus used as an inhibitor along with substrate to reduce background alkaline phosphatase activity in biomedical assays involving detection signal amplification by intestinal alkaline phosphatase, for example in in situ hybridization or Western blot protocols.
It is used to immobilize the nematode C. elegans on glass slides for imaging and dissection.[42]
In a C. elegans behavioral assay, analyzing the time course of paralysis provides information about the neuromuscular junction. Levamisole acts as an acetylcholine receptor agonist, which leads to muscle contraction. Continuing activation leads to paralysis. The time course of paralysis provides information about the acetylcholine receptors on the muscle. For example, mutants with fewer acetylcholine receptors may paralyze slower than wild type.[43]
Research
It has also been studied as a method to stimulate the immune system as part of the treatment of cancer.[44] It has also shown some efficacy in the treatment of nephrotic syndrome in children.[45]
References
- 1 2 3 4 "Levamisole Advanced Patient Information - Drugs.com". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- 1 2 3 4 5 6 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 86, 590. hdl:10665/44053. ISBN 9789241547659.
- ↑ Ritter, James M.; Flower, Rod; Henderson, Graeme; Loke, Yoon Kong; Rang, Humphrey P. (2020). "56. Antihelminthic drugs". Rang & Dale's Pharmacology. Elsevier. pp. 710–715. ISBN 978-0-7020-7448-6. Archived from the original on 2021-08-28. Retrieved 2021-10-12.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 November 2020. Retrieved 21 September 2020.
- ↑ Keiser J, Utzinger J (April 2008). "Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis". JAMA. 299 (16): 1937–48. doi:10.1001/jama.299.16.1937. PMID 18430913.
- ↑ Prevenier, Martha Howelland Walter (2001). From reliable sources : an introduction to historical methods (1. publ. ed.). Ithaca: Cornell university press. p. 77. ISBN 9780801485602. Archived from the original on 2017-09-10.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Levamisole". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 1 December 2016.
- ↑ Taylor, M. A.; Coop, R. L.; Wall, R. L. (2015). Veterinary Parasitology. John Wiley & Sons. p. 329. ISBN 9781119073673. Archived from the original on 2017-09-10.
- ↑ "Levamisole (Martindale: The Complete Drug Reference)". Lexicomp. Archived from the original on 21 December 2016. Retrieved 21 April 2014.
- ↑ Sanford, Shari (2007). "Levamisole Hydrochloride: Its application and usage in freshwater aquariums". Loaches Online. Archived from the original on 2009-03-01. Retrieved 2009-02-27.
- ↑ (Chirigos et al. (1969, 1973, 1975)).
- ↑ Scheinfeld N, Rosenberg JD, Weinberg JM (2004). "Levamisole in dermatology : a review". American Journal of Clinical Dermatology. 5 (2): 97–104. doi:10.2165/00128071-200405020-00004. PMID 15109274.
- ↑ Vanhoutte PM, Van Nueten JM, Verbeuren TJ, Laduron PM (January 1977). "Differential effects of the isomers of tetramisole on adrenergic neurotransmission in cutaneous veins of dog". The Journal of Pharmacology and Experimental Therapeutics. 200 (1): 127–40. PMID 189006. Archived from the original on 2021-08-28. Retrieved 2018-11-01.
- ↑ Przegaliński E, Bigajska K, Siwanowicz J (1980). "Psychopharmacological profile of dexamisole". Polish Journal of Pharmacology and Pharmacy. 32 (1): 21–9. PMID 7454609.
- ↑ "Levamisole" (PDF). DEA. Archived (PDF) from the original on 17 October 2013. Retrieved 21 April 2014.
- 1 2 3 Centers for Disease Control Prevention (CDC) (December 2009). "Agranulocytosis associated with cocaine use - four States, March 2008-November 2009". MMWR. Morbidity and Mortality Weekly Report. 58 (49): 1381–5. PMID 20019655. Archived from the original on 2018-10-09. Retrieved 2017-09-09.
- ↑ Chang A, Osterloh J, Thomas J (September 2010). "Levamisole: a dangerous new cocaine adulterant". Clinical Pharmacology and Therapeutics. 88 (3): 408–11. doi:10.1038/clpt.2010.156. PMID 20668440.
- ↑ "Cocaine powder: review of its prevalence, patterns of use and harm". Advisory Council on the Misuse of Drugs. 12 March 2015. Archived from the original on 10 September 2018. Retrieved 1 November 2018.
- ↑ Kouassi E, Caillé G, Léry L, Larivière L, Vézina M (1986). "Novel assay and pharmacokinetics of levamisole and p-hydroxylevamisole in human plasma and urine". Biopharmaceutics & Drug Disposition. 7 (1): 71–89. doi:10.1002/bdd.2510070110. PMID 3754161.
- ↑ Luyckx M, Rousseau F, Cazin M, Brunet C, Cazin JC, Haguenoer JM, Devulder B, Lesieur I, Lesieur D, Gosselin P, Adenis L, Cappelaere P, Demaille A (1982). "Pharmacokinetics of levamisole in healthy subjects and cancer patients". European Journal of Drug Metabolism and Pharmacokinetics. 7 (4): 247–54. doi:10.1007/bf03189626. PMID 7166176.
- ↑ Gutierrez, J.; Eisenberg, R.L.; Koval, N.J.; Armstrong, E.R.; Tharappel, J.; Hughes, C.G.; Tobin, T. (August 2010). "Pemoline and tetramisole 'positives' in English racehorses following levamisole administration". Irish Veterinary Journal. 63 (8): 498. doi:10.1186/2046-0481-63-8-498. PMC 4177197. PMID 21777496.
- ↑ Ho EN, Leung DK, Leung GN, Wan TS, Wong AS, Wong CH, Soma LR, Rudy JA, Uboh C, Sams R (April 2009). "Aminorex and rexamino as metabolites of levamisole in the horse". Analytica Chimica Acta. 638 (1): 58–68. doi:10.1016/j.aca.2009.02.033. PMID 19298880.
- ↑ J. Scarth et al. (2010) "The use of in vitro drug metabolism studies to complement, reduce and refine in vivo administrations in medication and doping control." Proceedings of the 18th International Conference of Racing Analyists and Veterinarians. pp 213-222
- ↑ Bertol E, Mari F, Milia MG, Politi L, Furlanetto S, Karch SB (July 2011). "Determination of aminorex in human urine samples by GC-MS after use of levamisole". Journal of Pharmaceutical and Biomedical Analysis. 55 (5): 1186–9. doi:10.1016/j.jpba.2011.03.039. PMID 21531521.
- ↑ Hess C, Ritke N, Broecker S, Madea B, Musshoff F (May 2013). "Metabolism of levamisole and kinetics of levamisole and aminorex in urine by means of LC-QTOF-HRMS and LC-QqQ-MS". Analytical and Bioanalytical Chemistry. 405 (12): 4077–88. doi:10.1007/s00216-013-6829-x. PMID 23436169.
- ↑ Vandamme TF, Demoustier M, Rollmann B (1995). "Quantitation of levamisole in plasma using high performance liquid chromatography". European Journal of Drug Metabolism and Pharmacokinetics. 20 (2): 145–9. doi:10.1007/bf03226369. PMID 8582440.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, pp.901-902. "Archived copy" (PDF). Archived from the original (PDF) on 2011-09-10. Retrieved 2011-01-22.
{{cite web}}: CS1 maint: archived copy as title (link). - ↑ Moisse, Katie (2011-06-23). "Cocaine Laced With Veterinary Drug Levamisole Eats Away at Flesh". ABC News. Archived from the original on 2011-06-25. Retrieved 2011-06-23.
- ↑ Doheny, Kathleen (Jun 1, 2010). "Contaminated Cocaine Can Cause Flesh to Rot". Yahoo!. Archived from the original on 7 June 2010. Retrieved 8 June 2010.
{{cite news}}: CS1 maint: postscript (link) - ↑ Kiley, Brendan (August 17, 2010). "The Mystery of the Tainted Cocaine". The Stranger. Archived from the original on December 11, 2010. Retrieved December 21, 2010.
- ↑ Zhu NY, Legatt DF, Turner AR (February 2009). "Agranulocytosis after consumption of cocaine adulterated with levamisole". Annals of Internal Medicine. 150 (4): 287–9. doi:10.7326/0003-4819-150-4-200902170-00102. PMID 19153405.
- ↑ Kinzie E (April 2009). "Levamisole found in patients using cocaine". Annals of Emergency Medicine. 53 (4): 546–7. doi:10.1016/j.annemergmed.2008.10.017. PMID 19303517.
- ↑ Menni, Silvano; Pistritto, Grazia; Gianotti, Raffaele; Ghio, Luciana; Edefonti, Alberto (1997). "Ear Lobe Bilateral Necrosis by Levamisole-Induced Occlusive Vasculitis in a Pediatric Patient". Pediatric Dermatology. 14 (6): 477–479. doi:10.1111/j.1525-1470.1997.tb00695.x.
- ↑ Bradford M, Rosenberg B, Moreno J, Dumyati G (June 2010). "Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole". Annals of Internal Medicine. 152 (11): 758–9. doi:10.7326/0003-4819-152-11-201006010-00026. PMID 20513844.
- ↑ "'Kate Plus Eight' is enough". The San Francisco Chronicle. September 29, 2009. Archived from the original on February 2, 2014. Retrieved January 21, 2014.
- ↑ Parascandola, Rocco (2010-06-18). "Ted Koppel's son, Andrew Koppel, overdosed on cocktail containing booze, heroin, cocaine and Valium". Daily News. New York. Archived from the original on 2010-06-22.
- ↑ Olding, Rachel (2014-01-12). "Young man's death highlights the tragic reality of online illegal drug stores". Sydney Morning Herald. Sydney. Archived from the original on 2014-01-13.
- ↑ Brendan Kiley (September 11, 2012). "Now Drug Dealers (Not Just Users) Are Testing Their Cocaine for Levamisole". The Stranger. Archived from the original on September 28, 2012. Retrieved September 14, 2012.
- ↑ J. Symoens et al. (1979). In Pharmacological and Biochemical Properties of Drug Substances, Vol. 2, (M. E. Goldberg, Ed.), pp. 407-464, Washington: American Pharmaceutical Association.
- ↑ Van Belle H (July 1976). "Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans". Clinical Chemistry. 22 (7): 972–6. PMID 6169. Archived from the original on 2020-01-25. Retrieved 2018-11-01.
- ↑ "Gonad Dissections | Schedl Lab". Archived from the original on 2014-05-17. Retrieved 2014-05-15. Schedl Lab Protocol for gonad dissections
- ↑ Rand JB (January 2007). "Acetylcholine". WormBook: 1–21. doi:10.1895/wormbook.1.131.1. PMC 4781110. PMID 18050502. Archived from the original on 2021-08-28. Retrieved 2018-11-01.
- ↑ Dillman RO (February 2011). "Cancer immunotherapy". Cancer Biotherapy & Radiopharmaceuticals. 26 (1): 1–64. doi:10.1089/cbr.2010.0902. PMID 21355777.
- ↑ Couderc A, Bérard E, Guigonis V, Vrillon I, Hogan J, Audard V, Baudouin V, Dossier C, Boyer O (December 2017). "[Treatments of steroid-dependent nephrotic syndrome in children]". Archives de Pediatrie. 24 (12): 1312–1320. doi:10.1016/j.arcped.2017.09.002. PMID 29146214.
External links
| External sites: |
|
|---|---|
| Identifiers: |