Dextrorphan
 | |
 | |
| Clinical data | |
|---|---|
| Other names | DXO |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.323 |
| Chemical and physical data | |
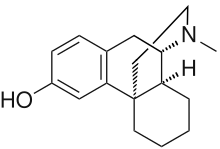
| Formula | C17H23NO |
| Molar mass | 257.377 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Dextrorphan (DXO) is a psychoactive drug of the morphinan class which acts as an antitussive or cough suppressant and dissociative hallucinogen. It is the dextrorotatory-stereoisomer of racemorphan, the levo-half being levorphanol. Dextrorphan is produced by O-demethylation of dextromethorphan by CYP2D6. Dextrorphan is an NMDA antagonist and contributes to the psychoactive effects of dextromethorphan.[1]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| NMDAR (MK-801) | 486–906 | Rat | [3] |
| σ1 | 118–481 | Rat | [3] |
| σ2 | 11,325–15,582 | Rat | [3] |
| MOR | 420 >1,000 | Rat Human | [3][6] |
| DOR | 34,700 | Rat | [3] |
| KOR | 5,950 | Rat | [3] |
| SERT | 401–484 | Rat | [3] |
| NET | ≥340 | Rat | [3] |
| DAT | >1,000 | Rat | [3] |
| 5-HT1A | >1,000 | Rat | [3] |
| 5-HT1B/1D | 54% at 1 μM | Rat | [3] |
| 5-HT2A | >1,000 | Rat | [3] |
| α1 | >1,000 | Rat | [3] |
| α2 | >1,000 | Rat | [3] |
| β | 35% at 1 μM | Rat | [3] |
| D2 | >1,000 | Rat | [3] |
| H1 | 95% at 1 μM | Rat | [3] |
| mAChRs | 100% at 1 μM | Rat | [3] |
| nAChRs | 1,300–29,600 (IC50) | Rat | [3] |
| VDSCs | ND | ND | ND |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | |||
The pharmacology of dextrorphan is similar to that of dextromethorphan (DXM). However, dextrorphan is much more potent as an NMDA receptor antagonist as well much less active as a serotonin reuptake inhibitor, but retains DXM's activity as a norepinephrine reuptake inhibitor.[7]
Pharmacokinetics
Dextrorphan has a notably longer elimination half-life than its parent compound, and therefore has a tendency to accumulate in the blood after repeated administration of normally dosed dextromethorphan formulations. It is further converted to 3-HM or glucuronidated.[8]
Society and culture
Legal status
Dextrorphan was formerly a Schedule I controlled substance in the United States, but was unscheduled on October 1, 1976.[9]
Research
Dextrorphan was under development for the treatment of stroke, and reached phase II clinical trials for this indication, but development was discontinued.[10]
Environmental presence
In 2021, dextrorphan was identified in >75% of sludge samples taken from 12 wastewater treatment plants in California. The same study associated dextrorphan with estrogenic activity by using predictive modelling, before observing it in in vitro. [11]
See also
References
- ↑ Zawertailo, L. A.; Kaplan, H. L.; Busto, U. E.; Tyndale, R. F.; Sellers, E. M. (Aug 1998). "Psychotropic Effects of Dextromethorphan are Altered by the CYP2D6 Polymorphism: A Pilot Study". Journal of Clinical Psychopharmacology. 18 (4): 332–337. doi:10.1097/00004714-199808000-00014. PMID 9690700.
- ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR (2016). "Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders". Pharmacol. Ther. 159: 1–22. doi:10.1016/j.pharmthera.2016.01.016. PMID 26826604.
- ↑ Werling LL, Keller A, Frank JG, Nuwayhid SJ (2007). "A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder". Exp. Neurol. 207 (2): 248–57. doi:10.1016/j.expneurol.2007.06.013. PMID 17689532. S2CID 38476281.
- ↑ Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR (2016). "Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta®) clinical use". Pharmacol. Ther. 164: 170–82. doi:10.1016/j.pharmthera.2016.04.010. PMID 27139517.
- ↑ Raynor K, Kong H, Mestek A, Bye LS, Tian M, Liu J, Yu L, Reisine T (1995). "Characterization of the cloned human mu opioid receptor". J. Pharmacol. Exp. Ther. 272 (1): 423–8. PMID 7815359.
- ↑ Pechnick, R. N.; Poland, R. E. (2004). "Comparison of the Effects of Dextromethorphan, Dextrorphan, and Levorphanol on the Hypothalamo-Pituitary-Adrenal Axis". Journal of Pharmacology and Experimental Therapeutics. 309 (2): 515–522. doi:10.1124/jpet.103.060038. PMID 14742749. S2CID 274504.
- ↑ Yu A, Haining RL (November 2001). "Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities?". Drug Metabolism and Disposition. 29 (11): 1514–20. PMID 11602530.
- ↑ DEA. "Lists of: Scheduling Actions Controlled Substances Regulated Chemicals" (PDF). Retrieved 2010-09-24.
- ↑ "Dextrorphan - AdisInsight".
- ↑ Gabrielle P. Black (2021). "Using Estrogenic Activity and Nontargeted Chemical Analysis to Identify Contaminants in Sewage Sludge". Environmental Science & Technology. 55 (10): 6729–6739. Bibcode:2021EnST...55.6729B. doi:10.1021/acs.est.0c07846. PMC 8378343. PMID 33909413.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||