Perzinfotel
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.222.780 |
| Chemical and physical data | |
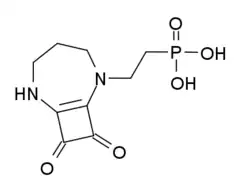
| Formula | C9H13N2O5P |
| Molar mass | 260.186 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Perzinfotel (EAA-090) is a drug which acts as a potent NMDA antagonist.[1] It has neuroprotective effects and has been investigated for the treatment of stroke,[2] but lacks analgesic effects.[3] Nevertheless, it shows a good safety profile compared to older drugs, although further development of this drug has been discontinued.[4]
Prodrugs were developed since the oral bioavailability of perzinfotel is only around 3-5%.[5]
References
- ↑ Kinney WA, Abou-Gharbia M, Garrison DT, Schmid J, Kowal DM, Bramlett DR, et al. (January 1998). "Design and synthesis of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)-ethyl]phosphonic acid (EAA-090), a potent N-methyl-D-aspartate antagonist, via the use of 3-cyclobutene-1,2-dione as an achiral alpha-amino acid bioisostere". Journal of Medicinal Chemistry. 41 (2): 236–46. doi:10.1021/jm970504g. PMID 9457246.
- ↑ Sun L, Chiu D, Kowal D, Simon R, Smeyne M, Zukin RS, et al. (August 2004). "Characterization of two novel N-methyl-D-aspartate antagonists: EAA-090 (2-[8,9-dioxo-2,6-diazabicyclo [5.2.0]non-1(7)-en2-yl]ethylphosphonic acid) and EAB-318 (R-alpha-amino-5-chloro-1-(phosphonomethyl)-1H-benzimidazole-2-propanoic acid hydrochloride)". The Journal of Pharmacology and Experimental Therapeutics. 310 (2): 563–70. doi:10.1124/jpet.104.066092. PMID 15075380. S2CID 25505657.
- ↑ Brandt MR, Cummons TA, Potestio L, Sukoff SJ, Rosenzweig-Lipson S (June 2005). "Effects of the N-methyl-D-aspartate receptor antagonist perzinfotel [EAA-090; [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)-ethyl]phosphonic acid] on chemically induced thermal hypersensitivity". The Journal of Pharmacology and Experimental Therapeutics. 313 (3): 1379–86. doi:10.1124/jpet.105.084467. PMID 15764736. S2CID 12989500.
- ↑ "Perzinfotel". Adis International Ltd. Springer Nature Switzerland AG.
- ↑ Baudy RB, Butera JA, Abou-Gharbia MA, Chen H, Harrison B, Jain U, et al. (February 2009). "Prodrugs of perzinfotel with improved oral bioavailability". Journal of Medicinal Chemistry. 52 (3): 771–8. doi:10.1021/jm8011799. PMID 19146418.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.