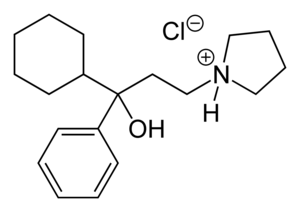
Procyclidine
 | |
| Names | |
|---|---|
IUPAC name
| |
| Clinical data | |
| Drug class | Anticholinergic[1] |
| Main uses | Parkinsonism, extrapyramidal symptoms due to antipsychotics[1] |
| Side effects | Dry mouth, blurry vision, nausea, constipation, lightheadedness[1] |
| Routes of use | By mouth, im, iv |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605037 |
| Pharmacokinetics | |
| Protein binding | ~100%-albumin |
| Elimination half-life | ~12 h |
| Chemical and physical data | |
| Formula | C19H30ClNO |
| Molar mass | 323.90 g·mol−1 |
Procyclidine, sold under the brand name Kemadrin among others, is a medication used to treat parkinsonism and extrapyramidal symptoms due to antipsychotics.[1] It improves tremors less than it improves rigidity.[1] It is taken by mouth or by injection into a vein or muscle.[1][2]
Common side effects include dry mouth, blurry vision, nausea, constipation, and lightheadedness.[1] Other side effects may include a fast heart rate, low blood pressure, urinary retention, confusion, and glaucoma.[1] Safety in pregnancy is unclear.[1] It is an anticholinergic.[1]
Procyclidine was approved for medical use in the United States in 1955.[1] It is available as a generic medication.[2] In the United Kingdom 100 tablets of 5 mg costs the NHS about £5 as of 2021.[2] It is no longer commercially available in the United States as of 2008.[3]
Medical uses
It is used in patients with parkinsonism and akathisia, and to reduce the side effects of antipsychotic treatment given for schizophrenia. Procyclidine is also a second-line drug for the treatment of Parkinson's disease. It improves tremor but not rigidity or bradykinesia.
Procyclidine is also sometimes used for the treatment of dystonia (but not tardive dyskinesia), a rare disorder that causes abnormal muscle contraction, resulting in twisting postures of limbs, trunk, or face.
Dosage
It is generally started at 2.5 mg three times per day by mouth and may be increased to 5 mg four times per day.[1]
Side effects
Side effects include nausea, constipation, urinary retention, blurred vision, anxiety, cognitive impairment, confusion, dizziness, gingivitis, hallucination, memory loss, rash and vomiting. [4]
Overdose
Signs of procyclidine overdose are those of an anticholinergic and include confusion, agitation and sleeplessness that can last up to or more than 24 hours. Pupils become dilated and unreactive to light. Tachycardia (fast heart beat), as well as auditory and visual hallucinations have also been reported.
Other known symptoms of overdose are: clumsiness or unsteadiness, being severely drowsy, having a severely dry mouth, nose, or throat, having an altered mood or other mental changes, seizures, being short of breath or having troubled breathing, a dry and warm, flushed skin.
A suspected overdose with severe life-threatening symptoms should immediately be brought to medical attention, where reversal can be attempted with physostigmine administered intravenously or subcutaneously.
Synthesis
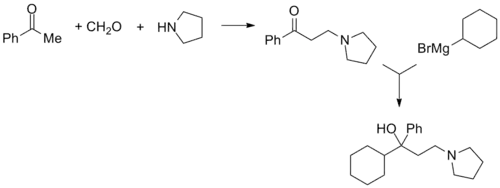
Procyclidine, 1-cyclohexyl-1-phenyl-3-pyrrolidinopropan-1-ol, is synthesized in exactly the same manner as was seen for trihexyphenidyl, except this time the linear synthesis begins with the preparation of 3-(1-pyrrolidino)propiophenone.
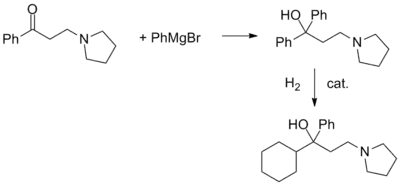
In an interesting variation, the ketone is first reacted with phenylmagnesium bromide. Catalytic hydrogenation of the carbinol thus obtained can be stopped after the reduction of only one aromatic ring.
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Procyclidine Monograph for Professionals". Drugs.com. Archived from the original on 23 October 2021. Retrieved 29 October 2021.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 432. ISBN 978-0857114105.
- ↑ "King discontinues Kemadrin tablets". MPR. 5 June 2008. Archived from the original on 4 December 2020. Retrieved 29 October 2021.
- ↑ "Procyclidine Hydrochloride". National Institute for Health and Care Excellence. Archived from the original on 2021-10-21. Retrieved 2020-07-06.
- ↑ Adamson DW, Barrett PA, Wilkinson S (1951). "11. Aminoalkyl tertiary carbinols and derived products. Part IV. Spasmolytics. Phenyl- and cyclohexylphenyl-carbinols". Journal of the Chemical Society (Resumed): 52. doi:10.1039/jr9510000052.
External links
| Identifiers: |
|---|
- British National Formulary (45 ed.). March 2003.