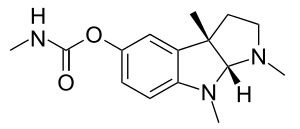
Physostigmine
 | |
| Names | |
|---|---|
| Trade names | Antilirium, Isopto Eserine, others |
| Other names | Eserine |
IUPAC name
| |
| Clinical data | |
| Drug class | Cholinesterase inhibitor[1] |
| Main uses | Reverse anticholinergic effects, hereditary ataxia[1] |
| Side effects | Stomach pain, small pupils, sweating, shortness of breath, seizures, cholinergic crisis, asystole[1] |
| Pregnancy category |
|
| Routes of use | intravenous, intramuscular, ophthalmic |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Major metabolite: Eseroline |
| Chemical and physical data | |
| Formula | C15H21N3O2 |
| Molar mass | 275.352 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Physostigmine, sold under the brand name Antilirium among others, is a medication used to reverse anticholinergic effects and to treat hereditary ataxia including Friedreich ataxia.[1] It is generally given by injection into a vein or muscle.[1]
Common side effects include nausea, stomach pain, small pupils, sweating, and shortness of breath.[1] Other side effects may include seizures, bronchospasm, cholinergic crisis, and asystole.[1] Safety in pregnancy is unclear.[2] It is a cholinesterase inhibitor.[1]
Physostigmine was originally isolated from the Calabar bean.[3] Its medical uses were discussed in the thesis of Thomas Richard Fraser at the University of Edinburgh in 1862.[3] It was first manufactured in 1935 by Percy Lavon Julian.[4] In the United States 2 mg costs about 80 USD as of 2021.[5]
Medical uses
Physostigmine is used to treat glaucoma and delayed gastric emptying. Because it enhances the transmission of acetylcholine signals in the brain and can cross the blood–brain barrier, physostigmine salicylate is used to treat anticholinergic poisoning (that is, poisoning by substances that interfere with the transmission of acetylcholine signaling, such as atropine, scopolamine, and other anticholinergic drug overdoses).[6] It is also used to reverse neuromuscular blocking. Physostigmine is the antidote of choice for Datura stramonium poisoning. It is also an antidote for Atropa belladonna poisoning, the same as for atropine.[7] It has also been used as an antidote for poisoning with GHB,[8] but is poorly effective and often causes additional toxicity, so is not a recommended treatment.[9]
It has been shown to improve long-term memory,[10] and was once explored as a therapy for Alzheimer's disease, but in clinical trials it was not shown to confer convincing benefits, and it led to very common moderate to severe side-effects such as nausea, vomiting, diarrhea, loss of appetite, abdominal pain, and tremors, resulting in a high rate of withdrawal.[11] Physostigmine's poor tolerability led to it being abandoned in favor of later acetylcholinesterase inhibitors, three of which are currently in use: donepezil, galantamine, and rivastigmine.[12]
Dosage
For reversing anticholinergic effects it may be used at a dose of 0.5 to 2 mg injected into a vein or muscle.[1] It may be given every 20 to 60 minutes as needed.[1]
Side effects
An overdose can cause cholinergic syndrome. Other side effects may include nausea, vomiting, diarrhea, anorexia, dizziness, headache, stomach pain, sweating, dyspepsia, and seizures.[13] The carbamate functional group readily hydrolyses in water, and in bodily conditions. The metabolite thus formed from physostigmine and some other alkaloids (e.g. cymserine) is eseroline, which research has suggested may be neurotoxic to humans.[14] Death can occur rapidly following overdose as a result of respiratory arrest and paralysis of the heart.
Pharmacology
Physostigmine acts by interfering with the metabolism of acetylcholine. It is a reversible inhibitor of acetylcholinesterase, the enzyme responsible for the breakdown of acetylcholine in the synaptic cleft of the neuromuscular junction.[15] It indirectly stimulates both nicotinic and muscarinic acetylcholine receptors. Physostigmine has an LD50 of 3 mg/kg in mice.
Bioactivity
Physostigmine functions as an acetylcholinesterase inhibitor. Its mechanism is to prevent the hydrolysis of acetylcholine by acetylcholinesterase at the transmitted sites of acetylcholine. This inhibition enhances the effect of acetylcholine, making it useful for the treatment of cholinergic disorders and myasthenia gravis. More recently, physostigmine has been used to improve the memory of Alzheimer's patients due to its potent anticholinesterase activity. However, its drug form, physostigmine salicylate, has poor bioavailability.
Physostigmine also has a miotic function, causing pupillary constriction. It is useful in treating mydriasis. Physostigmine also increases outflow of the aqueous humor in the eye, making it useful in the treatment of glaucoma.
Synthesis
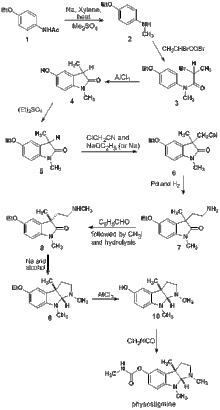
Julian & Pikl (1935)
Physostigmine has two stereocenters—the two carbons where the five-membered rings join together—so any attempt at the total synthesis must pay attention to obtaining the correct stereoisomer. The 71 syntheses of physostigmine yield 33 racemic mixtures and 38 products of a single enantiomer. The first total synthesis of physostigmine was achieved by Julian and Pikl in 1935.[16] The main goal of Julian's formal physostigmine synthesis was to prepare the key compound (L)-eseroline (compound 10 in the adjacent diagram), the conversion of which to physostigmine would be straightforward. In one of his earlier works[17] Julian synthesized the ring of physostigmine from 1-methyl-3-formyl-oxindole as starting material, which was discovered by Paul Friedländer. However, the starting material was expensive, and the reduction of a nitrile to an amine (similar to the reaction of compound 6 to give compound 7 in the diagram) with sodium and alcohol did not proceed in good yield. In his second work “Studies in the Indole Series III,” he had improved the yield of amine from nitrile significantly by using palladium and hydrogen. Although he succeeded in the synthesis of the target compound, the route had several drawbacks. First, the chemical resolution of compound 8 is unreliable, and the chemical resolution of rac-eserethole gives optically pure product only after eight recrystallizations of its tartrate salt. Second, the reductive amination going from compound 8 to compound 9 requires a large amount of Na. In the years since this initial work, many other groups have used a variety of approaches to construct the ring system and showcase new synthetic methods.
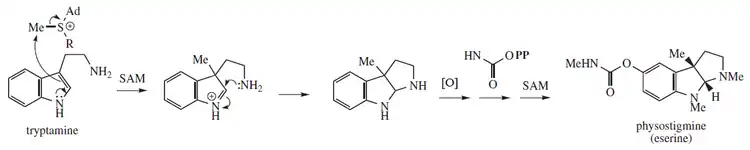
Biosynthesis
Physostigmine biosynthesis is proposed from tryptamine methylation and post-heterocyclization catalyzed by an unknown enzyme:[18]
History
Calabar bean
The Efik people, living in Cross River State and the Ibibio people in Akwa Ibom State, in what is now the south-south of Nigeria, were the first to come in contact with physostigmine, the active ingredient in the Calabar bean.[19] The Calabar bean, or chopping nut, was very prevalent in Efik culture as an ordeal poison. Individuals accused of witchcraft would drink the white, milky extract of the bean, made by crushing the bean in a mortar and soaking the remains in water. If the accused died, it was considered proof of their use of witchcraft. If they lived, usually due to vomiting up the poison, then they were declared innocent and sent free.[20]
It is also known as "eserine" from éséré, the West African name for the Calabar bean.
Western medicine
In 1846, European missionaries arrived in what was referred to as Old Calabar, now part of Nigeria. These missionaries wrote about the use of the Calabar bean as a test for witchcraft. These beans eventually made their way back to Scotland, the home of these particular missionaries, where in 1855 Robert Christison, a toxicologist, tested the toxicity of the poison on himself by eating one. He survived to document the experience. The bean was studied throughout the 1860s by a few different Edinburgh scientists, including Douglas Argyll Robertson who wrote a paper on the use of Calabar bean extract on the eye and was the first to use it medicinally, and Thomas Richard Fraser, who researched how to best extract the active principle, which was later determined to be physostigmine. Fraser also studied the antagonism between physostigmine and atropine extremely rigorously, at a time when the concept of antagonism had little if any experimental support. Fraser's research is still the basis of today's knowledge about the interactions between atropine and physostigmine at many different and specific doses.[21] Physostigmine's first use as a treatment for glaucoma was by Ludwig Laqueur in 1876. Laqueur himself suffered from glaucoma so, like Christison, he experimented on himself, although Laqueur was much more scientific and methodical in his self-treatment.
In the 1920s, Otto Loewi determined the biomechanical mechanism for the effects of physostigmine on the body. Loewi was studying how actions that we now consider to be controlled by the parasympathetic nervous system, were directed by chemicals. During his studies, Loewi discovered acetylcholine and that physostigmine acted by preventing acetylcholine inhibition. In 1936, Loewi was awarded the Nobel Prize for his work on discovering acetylcholine and biological chemical transmitters. More important discoveries surrounding physostigmine were made at the University of Edinburgh in 1925. Edgar Stedman and George Barger determined the structure of physostigmine using a method called chemical degradation. In 1935 Percy Lavon Julian was later the first to synthesize physostigmine. English scientist Robert Robinson was also working on the synthesis of physostigmine, but surprisingly Julian, a relatively unknown scientist at the time, was the successful one.[20]
In 1934, while working at St Alfege's Hospital in London, Dr Mary Walker discovered that a subcutaneous injection of physostigmine could temporarily reverse the muscle weakness found in patients suffering from myasthenia gravis. She had noted that the symptoms and signs of myasthenia were similar to those found in curare poisoning, and physostigmine was used as an antidote to curare poisoning at that time.[22] Her article explaining the first case of myasthenia gravis being successfully treated with physostigmine was published in The Lancet in June 1934.[23]
See also
References
- 1 2 3 4 5 6 7 8 9 10 "Physostigmine Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 28 October 2021.
- ↑ "Physostigmine (Antilirium) Use During Pregnancy". Drugs.com. Archived from the original on 3 December 2020. Retrieved 28 October 2021.
- 1 2 Doyle D (January 2009). "Sir Thomas Richard Fraser (1841–1920)" (PDF). JR Coll Physicians Edinb. 39: 283. Archived (PDF) from the original on 2021-01-25. Retrieved 2021-10-08.
- ↑ Sundberg, Richard J. (31 March 2017). The Chemical Century: Molecular Manipulation and Its Impact on the 20th Century. CRC Press. p. 331. ISBN 978-1-77188-367-2. Archived from the original on 28 October 2021. Retrieved 28 October 2021.
- ↑ "Physostigmine Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 24 January 2021. Retrieved 28 October 2021.
- ↑ Moore, Philip W.; Rasimas, J. J.; Donovan, J. W. (23 October 2014). "Physostigmine is the Antidote for Anticholinergic Syndrome". Journal of Medical Toxicology. 11 (1): 159–160. doi:10.1007/s13181-014-0442-z. PMC 4371033. PMID 25339374.
- ↑ Potter SO (1893). A Handbook of Materia Medica, Pharmacy and Therapeutics. London: P. Blakiston's. p. 53.
- ↑ Traub SJ, Nelson LS, Hoffman RS (2002). "Physostigmine as a treatment for gamma-hydroxybutyrate toxicity: a review". Journal of Toxicology. Clinical Toxicology. 40 (6): 781–7. doi:10.1081/CLT-120015839. PMID 12475191. S2CID 11134665.
- ↑ Zvosec DL, Smith SW, Litonjua R, Westfal RE (2007). "Physostigmine for gamma-hydroxybutyrate coma: inefficacy, adverse events, and review". Clinical Toxicology. 45 (3): 261–5. doi:10.1080/15563650601072159. PMID 17453877. S2CID 39337739.
- ↑ Krus et al. 1968
- ↑ Coelho F, Birks J (2001). "Physostigmine for Alzheimer's disease". The Cochrane Database of Systematic Reviews. 2 (2): CD001499. doi:10.1002/14651858.CD001499. PMC 8078195. PMID 11405996.
- ↑ Mehta M, Adem A, Sabbagh M (2012). "New acetylcholinesterase inhibitors for Alzheimer's disease". International Journal of Alzheimer's Disease. 2012: 728983. doi:10.1155/2012/728983. PMC 3246720. PMID 22216416.
- ↑ "Alzheimer Research Forum". Archived from the original on 2013-05-10. Retrieved 2021-10-08.
- ↑ Somani SM, Kutty RK, Krishna G (October 1990). "Eseroline, a metabolite of physostigmine, induces neuronal cell death". Toxicology and Applied Pharmacology. 106 (1): 28–37. doi:10.1016/0041-008X(90)90102-Z. PMID 2251681. Archived from the original on 2021-01-21. Retrieved 2021-10-08.
- ↑ Katzung BG, Masters S, Trever A (2009). Basic and Clinical Pharmacology. McGraw Hill. p. 110. ISBN 978-0-07-160405-5.
- ↑ Julian PL, Pikl J (1935). "Studies in the Indole Series. III. On the Synthesis of Physostigmine". Journal of the American Chemical Society. 57 (3): 539–544. doi:10.1021/ja01306a046.
- ↑ Julian PL, Pikl J, Boggess D (1934). "Studies in the Indole Series. II. Alkylation of 1-Methyl-3-Formyloxindole and a Synthesis of the Basic Ring Structure of Physostigmine". Journal of the American Chemical Society. 56 (8): 1797–1801. doi:10.1021/ja01323a046.
- ↑ Medicinal Natural Products.Dewick. 3rd edition
- ↑ Roberts MF, Wink M (1998). Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Plenum Press. p. 38. ISBN 978-1-4419-3263-1.
- 1 2 Scheindlin S (February 2010). "Episodes in the story of physostigmine". Molecular Interventions. 10 (1): 4–10. doi:10.1124/mi.10.1.1. PMID 20124558.
- ↑ Proudfoot A (2006). "The early toxicology of physostigmine: a tale of beans, great men and egos". Toxicological Reviews. 25 (2): 99–138. doi:10.2165/00139709-200625020-00004. PMID 16958557. S2CID 28243177.
- ↑ "Dr Mary Walker – A Pioneer in the Treatment of Myasthenia Gravis". MG -association UK. Archived from the original on 7 December 2008. Retrieved 23 November 2008.
- ↑ Walker MB (1934). "Treatment of myasthenia gravis with physostigmine". Lancet. 1 (5779): 1200–1201. doi:10.1016/S0140-6736(00)94294-6.
External links
| Identifiers: |
|---|