Potassium permanganate
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Potassium manganate(VII) | |
| Other names
Potassium permanganate Chameleon mineral Condy's crystals Permanganate of potash Hypermangan | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1490 |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
KMnO4 |
| Molar mass | 158.034 g/mol |
| Appearance | Purplish-bronze-gray needles Magenta–rose in solution[1] |
| Odor | odorless |
| Density | 2.7 g/cm3[2]: 4.83 |
| Melting point | 240 °C (464 °F; 513 K) (decomposes) |
Solubility in water |
76 g/L (25 °C)[2] 250 g/L (65 °C) |
| Solubility | decomposes in alcohol and organic solvents |
Magnetic susceptibility (χ) |
+20.0·10−6 cm3/mol[2]: 4.134 |
Refractive index (nD) |
1.59 |
| Structure[3] | |
Crystal structure |
Orthorhombic, oP24 |
Space group |
Pnma, No. 62 |
Lattice constant |
a = 0.909 nm, b = 0.572 nm, c = 0.741 nm |
Formula units (Z) |
4 |
| Thermochemistry | |
Heat capacity (C) |
119.2 J/mol K |
Std molar entropy (S⦵298) |
171.7 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−813.4 kJ/mol |
Gibbs free energy (ΔfG⦵) |
-713.8 kJ/mol |
| Pharmacology | |
| D08AX06 (WHO) V03AB18 (WHO) | |
| Hazards | |
| GHS pictograms |    |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1090 mg/kg (oral, rat)[4] |
| Related compounds | |
Other anions |
Potassium manganate |
Other cations |
Sodium permanganate Ammonium permanganate Calcium permanganate Silver permanganate |
Related compounds |
Manganese heptoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Legal | |
|---|---|
| License data | |
Potassium permanganate is an inorganic compound with the chemical formula KMnO4 and composed of K+ and MnO−
4. It is a purplish-black crystalline salt, that dissolves in water to give intensely pink or purple solutions.
Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the WHO Model List of Essential Medicines.[5] In 2000, worldwide production was estimated at 30,000 tonnes.[5]
Uses
Almost all applications of potassium permanganate exploit its oxidizing properties.[6] As a strong oxidant that does not generate toxic byproducts, KMnO4 has many niche uses.
Medical uses
Potassium permanganate is used for a number of skin conditions.[7] This includes fungal infections of the foot, impetigo, pemphigus, superficial wounds, dermatitis, and tropical ulcers.[8][7] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[5]
.jpg.webp) Potassium permanganate/therapeutic solution
Potassium permanganate/therapeutic solution.jpg.webp) Potassium permanganate/concentrated solution
Potassium permanganate/concentrated solution
Water treatment
Potassium permanganate is used extensively in the water treatment industry. It is used as a regeneration chemical to remove iron and hydrogen sulfide (rotten egg smell) from well water via a "Manganese Greensand" Filter. "Pot-Perm" is also obtainable at pool supply stores and is used additionally to treat waste water. Historically it was used to disinfect drinking water[9][10] and can turn the water pink.[11] It currently finds application in the control of nuisance organisms such as zebra mussels in fresh water collection and treatment systems.[12]
Synthesis of organic compounds

A major application of KMnO4 is as a reagent for the synthesis of organic compounds.[13] Significant amounts are required for the synthesis of ascorbic acid, chloramphenicol, saccharin, isonicotinic acid, and pyrazinoic acid.[6]
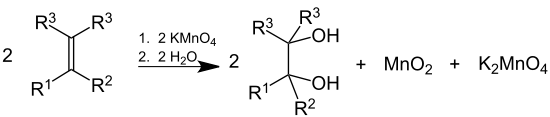
KMnO4 is used in qualitative organic analysis to test for the presence of unsaturation. It is sometimes referred to as Baeyer's reagent after the German organic chemist Adolf von Baeyer . The reagent is an alkaline solution of potassium permanganate. Reaction with double or triple bonds (-C=C- or -C≡C-) causes the color to fade from purplish-pink to brown. Aldehydes and formic acid (and formates) also give a positive test.[14] The test is antiquated.
Analytical use
Potassium permanganate can be used to quantitatively determine the total oxidizable organic material in an aqueous sample. The value determined is known as the permanganate value. In analytical chemistry, a standardized aqueous solution of KMnO4 is sometimes used as an oxidizing titrant for redox titrations (permanganometry). As potassium permanganate is titrated, the solution becomes a light shade of magenta, which darkens as excess of the titrant is added to the solution. In a related way, it is used as a reagent to determine the Kappa number of wood pulp. For the standardization of KMnO4 solutions, reduction by oxalic acid is often used.[15]
Aqueous, acidic solutions of KMnO4 are used to collect gaseous mercury in flue gas during stationary source emissions testing.[16]
In histology, potassium permanganate was used as a bleaching agent.[17][18]
Fruit preservation
Ethylene absorbents extend storage time of bananas even at high temperatures. This effect can be exploited by packing bananas in polyethylene together with potassium permanganate. By removing ethylene by oxidation, the permanganate delays the ripening, increasing the fruit's shelf life up to 4 weeks without the need for refrigeration.[19][20][21]
Survival kits
Potassium permanganate is sometimes included in survival kits: as a hypergolic fire starter (when mixed with glycerol antifreeze from a car radiator;[22][23][24] as a water sterilizer - and for creating distress signals on snow.[25]
Fire service
Potassium permanganate is added to "plastic sphere dispensers" to create backfires, burnouts, and controlled burns. Polymer spheres resembling ping-pong balls containing small amounts of permanganate are injected with ethylene glycol and projected towards the area where ignition is desired, where they spontaneously ignite seconds later.[26][27] Both handheld[27] and helicopter-[26] or boat-mounted[27] plastic sphere dispensers are used.
Other uses
Potassium permanganate is one of the principal chemicals used in the film and television industries to "age" props and set dressings. Its ready conversion to brown MnO2 creates "hundred-year-old" or "ancient" looks on Hessian cloth (burlap), ropes, timber, and glass.[28]
Potassium permanganate can be used to oxidize cocaine paste to purify it and increase its stability. This led to the Drug Enforcement Administration launching Operation Purple in 2000, with the goal of monitoring the world supply of potassium permanganate; however, potassium permanganate derivatives and substitutes were soon used thereafter to avoid the operation.[29]
In agricultural chemistry, it is used for estimation of available nitrogen in soil.
History
In 1659, Johann Rudolf Glauber fused a mixture of the mineral pyrolusite (manganese dioxide, MnO2) and potassium carbonate to obtain a material that, when dissolved in water, gave a green solution (potassium manganate) which slowly shifted to violet and then finally red.[30] This report represents the first description of the production of potassium permanganate.[31] Just under 200 years later, London chemist Henry Bollmann Condy had an interest in disinfectants; he found that fusing pyrolusite with sodium hydroxide (NaOH) and dissolving it in water produced a solution with disinfectant properties. He patented this solution, and marketed it as 'Condy's Fluid'. Although effective, the solution was not very stable. This was overcome by using potassium hydroxide (KOH) rather than NaOH. This was more stable, and had the advantage of easy conversion to the equally effective potassium permanganate crystals. This crystalline material was known as 'Condy's crystals' or 'Condy's powder'. Potassium permanganate was comparatively easy to manufacture, so Condy was subsequently forced to spend considerable time in litigation to stop competitors from marketing similar products.[32]
Early photographers used it as a component of flash powder. It is now replaced with other oxidizers, due to the instability of permanganate mixtures.
Preparation
Potassium permanganate is produced industrially from manganese dioxide, which also occurs as the mineral pyrolusite. In 2000, worldwide production was estimated at 30,000 tonnes.[6] The MnO2 is fused with potassium hydroxide and heated in air or with another source of oxygen, like potassium nitrate or potassium chlorate.[6] This process gives potassium manganate:
- 2 MnO2 + 4 KOH + O2 → 2 K2MnO4 + 2 H2O
(With sodium hydroxide, the end product is not sodium manganate but an Mn(V) compound, which is one reason the potassium permanganate is more commonly used than sodium permanganate. Furthermore, the potassium salt crystallizes better.[6])
The potassium manganate is then converted into permanganate by electrolytic oxidation in alkaline media:
- 2 K2MnO4 + 2 H2O → 2 KMnO4 + 2 KOH + H2
Other methods
Although of no commercial importance, potassium manganate can be oxidized by chlorine or by disproportionation under acid conditions.[33] The chlorine oxidation reaction is
- 2 K2MnO4 + Cl2 → 2 KMnO4 + 2 KCl
And the acid-induced disproportionation reaction may be written as
- 3 K2MnO4 + 4 HCl → 2 KMnO4 + MnO2 + 2 H2O + 4 KCl
A weak acid such as carbonic acid is sufficient for this reaction:
- 3 K2MnO4 + 2 CO2 → 2 KMnO4 + 2 K2CO3 + MnO2
Permanganate salts may also be generated by treating a solution of Mn2+ ions with strong oxidants such as lead dioxide (PbO2), sodium bismuthate (NaBiO3), or peroxydisulfate. Tests for the presence of manganese exploit the vivid violet color of permanganate produced by these reagents.
Structure
KMnO4 forms orthorhombic crystals with constants: a = 910.5 pm, b = 572.0 pm, c = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions.[34] In the solid (as in solution), each MnO4− centres are tetrahedral. The Mn–O distances are 1.62 Å.[35]
Reactions
Organic chemistry
Dilute solutions of KMnO4 convert alkenes into diols (glycols). This behaviour is also used as a qualitative test for the presence of double or triple bonds in a molecule, since the reaction decolorizes the initially purple permanganate solution and generates a brown precipitate (MnO2). In this context, it is sometimes called Baeyer's reagent. However, bromine serves better in measuring unsaturation (double or triple bonds) quantitatively, since KMnO4, being a very strong oxidizing agent, can react with a variety of groups.
Under acidic conditions, the alkene double bond is cleaved to give the appropriate carboxylic acid:[36]
- CH3(CH2)17CH=CH2 + 2 KMnO4 + 3 H2SO4 → CH3(CH2)17COOH + CO2 + 4 H2O + K2SO4 + 2 MnSO4
Potassium permanganate oxidizes aldehydes to carboxylic acids, such as the conversion of n-heptanal to heptanoic acid:[37]
- 5 C6H13CHO + 2 KMnO4 + 3 H2SO4 → 5 C6H13COOH + 3 H2O + K2SO4 + 2 MnSO4
Even an alkyl group (with a benzylic hydrogen) on an aromatic ring is oxidized, e.g. toluene to benzoic acid.[38]
- 5 C6H5CH3 + 6 KMnO4 + 9 H2SO4 → 5 C6H5COOH + 14 H2O + 3 K2SO4 + 6 MnSO4
Glycols and polyols are highly reactive toward KMnO4. For example, addition of potassium permanganate to an aqueous solution of sugar and sodium hydroxide produces the chemical chameleon reaction, which involves dramatic color changes associated with the various oxidation states of manganese. A related vigorous reaction is exploited as a fire starter in survival kits. For example, a mixture of potassium permanganate and glycerol or pulverized glucose ignites readily.[22] Its sterilizing properties are another reason for inclusion of KMnO4 in a survival kit.
By itself, potassium permanganate does not dissolve in many organic solvents. If an organic solution of permanganate is desired, "purple benzene" may be prepared, either by treating a two phase mixture of aqueous potassium permanganate and benzene with a quaternary ammonium salt,[39] or by sequestering the potassium cation with a crown ether.[40]
Reaction with acids
The reaction of permanganate with concentrated hydrochloric acid gives chlorine. The Mn-containing products from redox reactions depend on the pH. Acidic solutions of permanganate are reduced to the faintly pink manganese(II) ion (Mn2+) and water. In neutral solution, permanganate is only reduced by three electrons to give manganese dioxide (MnO2), wherein manganese is in a +4 oxidation state. This is the material that stains one's skin when handling KMnO4. KMnO4 spontaneously reduces in an alkaline solution to green K2MnO4, wherein manganese is in the +6 oxidation state.
A curious reaction occurs upon addition of concentrated sulfuric acid to potassium permanganate. Concentrated sulfuric acid reacts with KMnO4 to give Mn2O7, which can be explosive.[41] Although no reaction may be apparent, the vapor over the mixture will ignite paper impregnated with alcohol. Potassium permanganate and sulfuric acid react to produce some ozone, which has a high oxidizing power and rapidly oxidizes the alcohol, causing it to combust. As the reaction also produces explosive Mn2O7, this should only be attempted with great care.[42][43]
Thermal decomposition
Solid potassium permanganate decomposes when heated:
- 2 KMnO4 → K2MnO4 + MnO2(s) + O2
Here, the oxidation state of manganese changes as the potassium permanganate (oxidation state +7) decomposes to potassium manganate (oxidation state +6) and manganese dioxide (oxidation state +4). Oxygen gas is also liberated.
Safety and handling
Potassium permanganate poses risks as an oxidizer.[44] Contact with skin will result in a long lasting brown stain.[45]
References
- ↑ Burriel, F.; Lucena, F.; Arribas, S. and Hernández, J. (1985), Química Analítica Cualitativa, p. 688, ISBN 84-9732-140-5.
- 1 2 3 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. ISBN 978-1439855119.
- ↑ Hocart R., Sicaud, Mathieu (1945) C R Hebd Seances Acad Sci., 221, 261–263
- ↑ Chambers, Michael. "ChemIDplus – 7722-64-7 – VZJVWSHVAAUDKD-UHFFFAOYSA-N – Potassium permanganate [USP:JAN] – Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.sis.nlm.nih.gov. Archived from the original on 13 August 2014. Retrieved 9 May 2018.
- 1 2 3 World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 3 4 5 Reidies, Arno H. (2002). "Manganese Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_123.
- 1 2 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 295, 300. hdl:10665/44053. ISBN 9789241547659.
- ↑ British Medical Association; Royal Pharmaceutical Society (2015). British national formulary (69 ed.). p. 840. ISBN 9780857111562.
- ↑ Assembly of Life Sciences (U.S.). Safe Drinking Water Committee (1977). Drinking water and health, Volume 2. National Academies Press. p. 98. ISBN 978-0-309-02931-5. Retrieved 2016-09-13.
- ↑ Downey, Robyn and Barrington, Mike (28 February 2005) "Red faces over pink water", The Northern Advocate.
- ↑ "Onoway apologizes for 'alarming' pink tap water". CBC News. 7 March 2017. Archived from the original on 7 March 2017. Retrieved 8 March 2017.
- ↑ EPA Guidance Manual Alternative Disinfectants and Oxidants Archived 2016-10-01 at the Wayback Machine. epa.gov
- ↑ Fatiadi, A. (1987). "The Classical Permanganate Ion: Still a Novel Oxidant in Organic Chemistry". Synthesis. 1987 (2): 85–127. doi:10.1055/s-1987-27859.
- ↑ "Chemistry and Biochemistry". www.chemistry.ccsu.edu. Archived from the original on 24 January 2013. Retrieved 9 May 2018.
- ↑ Kovacs KA, Grof P, Burai L, Riedel M (2004). "Revising the Mechanism of the Permanganate/Oxalate Reaction". J. Phys. Chem. A. 108 (50): 11026. Bibcode:2004JPCA..10811026K. doi:10.1021/jp047061u.
- ↑ Code of Federal Regulations(7-1-07) Edition, Title 40, Part 60, Appendix A-8, Method 29, Section 7.3.1
- ↑ Picken, MM. (Apr 2010). "Amyloidosis-where are we now and where are we heading?". Arch Pathol Lab Med. 134 (4): 545–51. doi:10.5858/134.4.545. PMID 20367306.
- ↑ Murphy CL, Eulitz M, Hrncic R, Sletten K, Westermark P, Williams T, Macy SD, Wooliver C, Wall J, Weiss DT, Solomon A (2001). "Chemical typing of amyloid protein contained in formalin-fixed paraffin-embedded biopsy specimens". Am. J. Clin. Pathol. 116 (1): 135–42. doi:10.1309/TWBM-8L4E-VK22-FRH5. PMID 11447744.
- ↑ Scott, KJ, McGlasson WB and Roberts EA (1970). "Potassium Permanganate as an Ethylene Absorbent in Polyethylene Bags to Delay the Ripening of Bananas During Storage". Australian Journal of Experimental Agriculture and Animal Husbandry. 10 (43): 237. doi:10.1071/EA9700237.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Scott KJ, Blake, JR, Stracha, G, Tugwell, BL and McGlasson WB (1971). "Transport of Bananas at Ambient Temperatures using Polyethylene Bags". Tropical Agriculture (Trinidad). 48: 163–165.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Scott, KJ & Gandanegara, S (1974). "Effect of Temperature on the Storage Life of bananas Held in Polyethylene Bags with an Ethylene Absorbent". Tropical Agriculture (Trinidad). 51: 23–26.
- 1 2 Gillis, Bob & Labiste, Dino. "Fire by Chemical Reaction". Archived from the original on 2015-09-24.
- ↑ "pssurvival.com" (PDF). pssurvival.com. Archived (PDF) from the original on 4 August 2016. Retrieved 9 May 2018.
- ↑ "Making Fire with Potassium Permanganate and Glycerin". thesurvivalcache. 3 November 2012. Archived from the original on 13 May 2016. Retrieved 13 September 2016.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ↑ "Distress Signals". Evening Post. CXXI (107): 5. 7 May 1936. Archived from the original on 5 November 2011.
- 1 2 "Missoula Technology and Development Center: Aerial Ignition, Plastic Sphere Dispenser Description". www.fs.fed.us. Archived from the original on 26 April 2016. Retrieved 9 May 2018.
- 1 2 3 ""Wetland Warrior"". Dirty Jobs. Season 6. Episode 2.
- ↑ Brody, Ester (February 2000). "Victor DeLor contractor profile". PaintPRO. 2 (1). Archived from the original on 2008-07-23. Retrieved 2009-11-12.
One of the techniques DeLor is known for among designers and clients is the special effects he creates with various chemical solutions. When applied to wood surfaces, these chemicals give a weathered appearance to new wood. ... To achieve the aesthetic on interior surfaces, DeLor often uses a mixture of water and potassium permanganate, a dry powder chemical.
- ↑ Gosmer, Kim; Vammen, Morten (March 10, 2014). "The Terrifying Substances People Put in Cocaine". Vice Media. Archived from the original on July 26, 2020. Retrieved June 2, 2021.
- ↑ Glauber, Johann Rudolph, Prosperitas Germaniae (The prosperity of Germany), part 3 (Amsterdam, (Netherlands): Johann Jansson, 1659), pp. 93–94. From pp. 93–94: " … donec tandem Magnesiam istam nitro fixo permixtam, in crucibulo forti coctione a nitro reseratam vidi, unde elegans color purpureus provenit, massam hanc effusam in pulvere redegi, aqua calida extraxi, per filtrum liquorem transmisi. Tandem vero elegantissimum purpureum, igneumque liquorem accepi, qui fere singulis horis in frigore tantummodo consistens colorem permutavit, sic ut jam viridis, jam caerulei, jam sanguinei coloris sponte sua factus sit, mox iterum alios elegantissimos colores receperit." ( … until finally I saw [that] by mixing that magnesia [i.e., magnesia nigra, pyrolusite, the ore containing manganese dioxide ] with fixed niter [i.e., inert niter, potassium carbonate], by cooking [it] in a strong crucible, [the colored compound was] released by the niter, whence a fine purple color arises ; this mass [was] poured out, reduced to powder, extracted with hot water, [and] the solution passed through a filter. Then I got a truly most elegant, purple, and fiery solution, which nearly every hour ([while] standing just in the cold) changed color, so that it was spontaneously made now green, now blue, now red in color; soon again it received other most elegant colors.) Available at: Bavarian State Library Archived 2016-12-20 at the Wayback Machine English translation: Glauber, John Rudolph with Christopher Packe, trans., The Works of the Highly Experienced and Famous Chymist, John Rudolph Glauber: … (London, England: Thomas Millbourn, 1689), p. 353. Archived 2012-01-07 at the Wayback Machine The reaction that produced the color changes that Glauber observed in his solution of potassium permanganate and potassium manganate (K2MnO4) is now known as the "chemical chameleon".
- ↑ Weeks, Mary Elvira with Henry M. Leicester, ed., Discovery of the Elements, 6th ed. (Easton, Pennsylvania: Journal of Chemical Education, 1956), pp. 172–173.
- ↑ "Important Trade Mark Case". Otago Witness. 2 (2420): 53. 2 August 1900. Archived from the original on 4 March 2016.
- ↑ Walton, H. F. (1948) Inorganic Preparations. New York. pp. 150–151
- ↑ "Handbook of Preparative Inorganic Chemistry" Brauer, E., Ed.; Academic: New York, 1963.
- ↑ Palenik, Gus J. (1967). "Crystal structure of potassium permanganate". Inorg. Chem. 6 (3): 503–507. doi:10.1021/ic50049a015.
- ↑ Donald G. Lee, Shannon E. Lamb, and Victor S. Chang (1990). "Carboxylic Acids from the Oxidation of Terminal Alkenes by Permanganate: Nonadecanoic Acid". Organic Syntheses.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 7, p. 397 - ↑ Ruhoff, John R. "n-Heptanoic acid". Organic Syntheses.; Collective Volume, vol. 2, p. 315
- ↑ Gardner KA, Mayer JM (1995). "Understanding C-H Bond Oxidations: H· and H- Transfer in the Oxidation of Toluene by Permanganate". Science. 269 (5232): 1849–51. Bibcode:1995Sci...269.1849G. doi:10.1126/science.7569922. PMID 7569922.
- ↑ Arthur W. Herriott (1977). "Purple benzene: Solubilization of anions in organic solvents". J. Chem. Educ. 54 (4): 229. Bibcode:1977JChEd..54Q.229H. doi:10.1021/ed054p229.1.
- ↑ Doheny, Anthony J., Jr. & Ganem, Bruce (1980). "Purple benzene revisited". J. Chem. Educ. 57 (4): 308. Bibcode:1980JChEd..57..308D. doi:10.1021/ed057p308.1.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Cotton, F. A.; Wilkinson, G.; Murillo, C. A. and Bochmann, M. (1999). Advanced Inorganic Chemistry, 6th Edition. Wiley-VCH. ISBN 0-471-19957-5
- ↑ Barthel, H. & Duvinage, B. (2000). "Clemens Winkler. His Experiments with Ozone in 1892". Praxis der Naturwissenschaften, Chemie. 49: 18ff.
- ↑ Dzhabiev, T. S.; Denisov, N. N.; Moiseev, D. N. & Shilov, A. E. (2005). "Formation of Ozone During the Reduction of Potassium Permanganate in Sulfuric Acid Solutions". Russian Journal of Physical Chemistry. 79: 1755–1760.
- ↑ Bretherick, L.; Urben, P. G.; Pitt, Martin John (2007), Bretherick's Handbook of Reactive Chemical Hazards, vol. 1 (7 ed.), Elsevier Academic Press, pp. 1811–7, ISBN 978-0-12-373945-2
- ↑ Griffin, Sharin. "How to Remove Potassium Permanganate". livestrong.com. Archived from the original on 13 April 2018. Retrieved 9 May 2018.
External links
- International Chemical Safety Card 0672
- "Potassium permanganate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-21. Retrieved 2021-06-02.
- National Pollutant Inventory: Manganese and compounds Fact Sheet Archived 2021-06-05 at the Wayback Machine
- The use of potassium permanganate in fish ponds
