Phototrexate
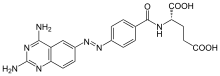 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C20H19N7O5 |
| Molar mass | 437.416 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Phototrexate is a photochromic antifolate drug developed at the Institute for Bioengineering of Catalonia (IBEC, The Barcelona Institute of Science and Technology). In particular, it is a photopharmacological agent[1][2] that behaves as light-regulated inhibitor of the dihydrofolate reductase (DHFR) enzyme.[3][4] Phototrexate is a photoisomerizable structural analogue of the chemotherapy agent methotrexate. It is also an example of "azologization".[5] Pharmacological effects of phototrexate can be switched on and off by UVA and visible light, respectively. Phototrexate is almost inactive in its trans configuration while it behaves as a potent antifolate in its cis configuration. It can also spontaneously self-deactivate in the dark.
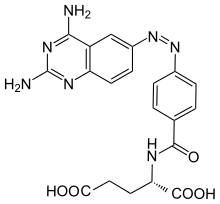
cis-Phototrexate
See also
References
- ↑ Velema WA, Szymanski W, Feringa BL (February 2014). "Photopharmacology: beyond proof of principle". Journal of the American Chemical Society. 136 (6): 2178–91. doi:10.1021/ja413063e. hdl:11370/d6714f52-c2c8-4e48-b345-238e98bcc776. PMID 24456115.
- ↑ Broichhagen J, Frank JA, Trauner D (July 2015). "A roadmap to success in photopharmacology". Accounts of Chemical Research. 48 (7): 1947–60. doi:10.1021/acs.accounts.5b00129. PMID 26103428.
- ↑ Matera C, Gomila AM, Camarero N, Libergoli M, Soler C, Gorostiza P (November 2018). "Photoswitchable Antimetabolite for Targeted Photoactivated Chemotherapy". Journal of the American Chemical Society. 140 (46): 15764–15773. doi:10.1021/jacs.8b08249. hdl:2445/126377. PMID 30346152.
- ↑ Mashita T, Kowada T, Takahashi H, Matsui T, Mizukami S (June 2019). "Light-Wavelength-Based Quantitative Control of Dihydrofolate Reductase Activity by Using a Photochromic Isostere of an Inhibitor". ChemBioChem. 20 (11): 1382–1386. doi:10.1002/cbic.201800816. PMID 30656808. S2CID 58567138.
- ↑ Schoenberger M, Damijonaitis A, Zhang Z, Nagel D, Trauner D (July 2014). "Development of a new photochromic ion channel blocker via azologization of fomocaine". ACS Chemical Neuroscience. 5 (7): 514–8. doi:10.1021/cn500070w. PMC 4102962. PMID 24856540.
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.