Plasmodium vivax
| Plasmodium vivax | |
|---|---|
 | |
| Mature P. vivax trophozoite | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | TSAR |
| Clade: | SAR |
| Clade: | Alveolata |
| Phylum: | Apicomplexa |
| Class: | Aconoidasida |
| Order: | Haemospororida |
| Family: | Plasmodiidae |
| Genus: | Plasmodium |
| Species: | P. vivax |
| Binomial name | |
| Plasmodium vivax (Grassi & Feletti, 1890) | |
| Synonyms[1][2] | |
| |
Plasmodium vivax (Plasmodium vivax Malaria[3]) is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria.[4] [2]
Although it is less virulent than Plasmodium falciparum, the deadliest of the five human malaria parasites P. vivax malaria infections can lead to severe disease and death, often due to splenomegaly (a pathologically enlarged spleen).[5][6] P. vivax is carried by the female Anopheles mosquito; the males do not bite.[7]
There is evidence that P. vivax is itself infected by viruses.[8]
Biology
Life cycle
Like all malaria parasites, P. vivax has a complex life cycle. It infects a definitive insect host, where sexual reproduction occurs, and an intermediate vertebrate host, where asexual amplification occurs. In P. vivax, the definitive hosts are Anopheles mosquitoes, while humans are the intermediate asexual hosts. During its life cycle, P. vivax assumes various different physical forms.[9][10][11][12]
- Sporozoite: Transfers infection from mosquito to human
- Immature trophozoites: Ring or signet-ring shaped
- Mature trophozoites: Very irregular and delicate; many pseudopodial processes seen. Presence of fine grains of brown pigment or hematin probably derived from the haemoglobin of the infected red blood cell.
- Schizonts: Is large and so the parasitized corpuscle becomes distended and larger than normal.
- Gametocytes: Round. P. vivax gametocytes are commonly found in human peripheral blood at about the end of the first week of parasitemia.
- Gametes: Formed from gametocytes in mosquitoes.
- Zygote: Formed from combination of gametes
- Oocyst: Contains zygote, develops into sporozoites
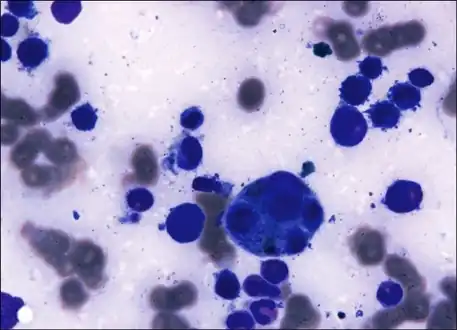 Bone marrow aspirate from the individual with Plasmodium vivax infection
Bone marrow aspirate from the individual with Plasmodium vivax infection Microphotographs of Plasmodium vivax in Giemsa-stained thin blood films. a, b ring stages, c–e young trophozoites, f–h amoeboid trophozoites, i young schizont, j–l growing schizonts, m developed schizont, n mature schizont, o young gametocyte, p macroga‑metocyte, r, q microgametocytes.[18]
Microphotographs of Plasmodium vivax in Giemsa-stained thin blood films. a, b ring stages, c–e young trophozoites, f–h amoeboid trophozoites, i young schizont, j–l growing schizonts, m developed schizont, n mature schizont, o young gametocyte, p macroga‑metocyte, r, q microgametocytes.[18]
Human infection
P. vivax human infection occurs when an infected mosquito feeds on a human. During feeding, the mosquito injects saliva, along with sporozoites, through the skin. A proportion of these sporozoites reach the liver. There they enter hepatic cells, on which they feed, and reproduce asexually, as described in the next section. This process gives rise to thousands of merozoites (plasmodial daughter cells) in the body.[19]
The incubation period of human infection usually ranges from ten to seventeen days and sometimes up to a year. Persistent liver stages allow relapse up to five years after elimination of red blood cell stages and clinical cure.[20][11]
Liver stage

The P. vivax sporozoite enters a hepatocyte and begins its exoerythrocytic schizogony stage. This is characterized by multiple rounds of nuclear division without cellular segmentation. After a number of nuclear divisions, the parasite cell will segment and merozoites are formed[22][11].
There are situations where some of the sporozoites do not immediately start to grow and divide after entering the hepatocyte, but remain in a dormant, hypnozoite stage for weeks or months. The duration of latency is thought to be variable from one hypnozoite to another and the factors that will eventually trigger growth are not known; this might explain how a single infection can be responsible for a series of waves of parasitaemia or "relapses".[23]
However, such recurrent parasitemia is probably being over-attributed to hypnozoite activation.[24] Two newly recognized, non-hypnozoite, probable contributing sources to recurrent peripheral P. vivax parasitemia are erythrocytic forms in bone marrow and the spleen.[25] Between 2018 and 2021, it was reported that vast numbers of non-circulating, non-hypnozoite parasites occur unobtrusively in tissues of P. vivax-infected people, with only a small proportion of the total parasite biomass present in the peripheral bloodstream. This finding supports an intellectually insightful, paradigm-shifting viewpoint, which had prevailed since 2011, that an unknown percentage of P. vivax recurrences are recrudescences (having a non-circulating or sequestered merozoite origin), and not relapses (which have a hypnozoite source). The recent discoveries concerning bodily parasite biomass distribution did not give rise to this new theory; it was pre-existing, as explained above.[26]
Erythrocytic cycle
P. vivax preferentially penetrates young red blood cells (reticulocytes), unlike Plasmodium falciparum which can invade erythrocytes. In order to achieve this, merozoites have two proteins at their apical pole (PvRBP-1 and PvRBP-2). The parasite uses the Duffy blood group antigens (Fy6) to penetrate red blood cells. This antigen does not occur in the majority of humans in West Africa [phenotype Fy (a-b-)]. As a result, P. vivax occurs less frequently in West Africa.[27]
The parasitised red blood cell is up to twice as large as a normal red cell and Schüffner's dots are seen on the infected cell's surface. Schüffner's dots have a spotted appearance, varying in color from light pink, to red, to red-yellow, as coloured with Romanovsky stains. The parasite within it is often wildly irregular in shape . Schizonts of P. vivax have up to twenty merozoites within them. It is rare to see cells with more than one parasite within them. Merozoites will only attach to immature blood cell and therefore it is unusual to see more than a very small percentage of all circulating erythrocytes parasitised.[28][11][29]
Mosquito stage

Parasite life cycle in mosquitoes includes all stages of sexual reproduction:[30][31]
Mosquito Infection and Gamete Formation

When a female Anopheles mosquito bites an infected person, gametocytes and other stages of the parasite are transferred to the mosquito stomach. Gametocytes ultimately develop into gametes, a process known as gametogony.Microgametocytes become very active. The cytoplasm develops long thin flagella like projections, then a nucleus enters into each one of these extensions. These cytoplasmic extensions later break off as mature male gametes . This process of formation of flagella-like microgametes or male gametes is known as exflagellation.Macrogametocytes show very little change. They develop a cone of reception at one side and becomes mature as macrogametocytes .[32][33][34][35]
Fertilization
Male gametes move actively in the stomach of mosquitoes in search of female gametes. Male gametes then enter into female gametes through the cone of reception. The complete fusion of 2 gametes results in the formation of zygote. Here, fusion of 2 dissimilar gametes occurs, known as anisogamy.The zygote remains inactive for sometime but it soon elongates, becomes vermiform and motile. It is now known as ookinete, the pointed ends of ookinete penetrate the stomach wall and come to lie below its outer epithelial layer. Here the zygote becomes spherical and develops a cyst wall around itself. The cyst wall is derived partly from the stomach tissues and partly produced by the zygote itself. At this stage, the zygote is known as an oocyst. The oocyst absorbs nourishment and grows in size. Oocysts protrude from the surface of stomach, giving it a blistered appearance.[36][37][38][39]
Sporogony
The oocyst nucleus divides repeatedly to form a large number of daughter nuclei. At the same time, the cytoplasm develops large vacuoles and forms numerous cytoplasmic masses. These cytoplasmic masses then elongate and a daughter nuclei migrates into each mass. The resulting sickle-shaped bodies are known as sporozoites, this phase of asexual multiplication is known as sporogony. The oocyst then bursts and sporozoites are released into the body cavity of mosquito. Sporozoites eventually reach the salivary glands of mosquito via its hemolymph, the mosquito now becomes infectious. Salivary glands of a single infected mosquito contain a large number of sporozoites.When the mosquito bites a healthy person, thousands of sporozoites are infected into the blood along with the saliva. It should be noted that in sporogony, protein S6 plays an important role.[40][41][42][43]
Taxonomy
P. vivax can be divided into two clades one that appears to have origins in the Old World and a second that originated in the New World.[44]
The distinction can be made on the basis of the structure of the A and S forms of the rRNA. A rearrangement of these genes appears to have occurred in the New World strains. It appears that a gene conversion occurred in an Old World strain and this strain gave rise to the New World strains. The timing of this event has yet to be established. At present both types of P. vivax circulate in the Americas. The monkey parasite - Plasmodium simium - is related to the Old World strains rather than to the New World strains.A specific name - Plasmodium collinsi - has been proposed for the New World strains but this suggestion has not been accepted to date.[45][46][47][48]
Horizontal gene transfer
It has been suggested that P. vivax has horizontally acquired genetic material from humans.[49]
Human disease
Signs and symptoms
Results from rupture of infected red blood cells, leading to fever. Infected red blood cells may also stick to each other and to walls of capillaries. Vessels plug up and deprive tissues of oxygen. Infection may also cause the spleen to enlarge.[30][11]
Unlike P. falciparum, P. vivax can populate the bloodstream, even before a patient shows symptoms, with sexual-stage parasites—the form ingested by mosquitoes prior to biting the next victim. Consequently, prompt treatment of symptomatic patients does not necessarily help stop an outbreak, as it does with falciparum malaria, in which fevers occur as sexual stages develop. Even when symptoms appear, because the disease is usually not immediately fatal, the parasite continues to multiply.[50]
Plasmodium vivax can cause a more unusual form of malaria with atypical symptoms. It has been known to debut with hiccups,[51] loss of taste, lack of fever, pain while swallowing, cough and urinary discomfort.[52] The parasite can lie dormant in the liver for days to years, causing no symptoms and remaining undetectable in blood tests. They form hypnozoites, a small stage that nestles inside an individual liver cell. This name derives from "sleeping organisms".[53] The hypnozoites allow the parasite to survive in more temperate zones, where mosquitoes bite only part of the year.[50]A single infectious bite can trigger six or more relapses a year, leaving patients more vulnerable to other diseases. Other infectious diseases, including falciparum malaria, appear to trigger relapses.[50]
Complications
Serious complications for malaria are dormant liver stage parasites, organ failures such as acute kidney failure. More complications of malaria can also be impairment of consciousness, neurological abnormalities, hypoglycemia and low blood pressures caused by cardiovascular collapse, clinical jaundice and or other vital organ dysfunctions and coagulation defects. The most serious complication ultimately being death.[54]
Diagnosis
In terms of the diagnosis it might be elicited by any of the following: recent travel history, enlarged spleen, fever, low number of platelets in the blood, and higher-than-normal levels of bilirubin in the blood combined with a normal level of white blood cells.[55]
Reports in 2016 and 2017 from countries where malaria is common suggest high levels of over diagnosis due to insufficient or inaccurate laboratory testing.[56][57][58]
Blood films are preferably made within 30 minutes of the blood draw and must certainly be made within an hour of the blood being drawn. Diagnosis can be done with the strip fast test of antibodies.[59][60]
Prevention
The main way to prevent malaria is through vector control. There are mostly three main forms that the vector can be controlled: (1) insecticide-treated mosquito nets, (2) indoor residual spraying and (3) antimalarial drugs. Long-lasting insecticidal nets (LLNs) are the preferred method of control because it is the most cost effective. The WHO is currently strategizing how to ensure that the net is properly maintained to protect people at risk. The second option is indoor residual spraying and has been proven effective if at least 80% of the homes are sprayed. However, such method is only effective for 3-6months. A drawback to these two methods, unfortunately, is that mosquito resistance against these insecticides has risen. National malaria control efforts are undergoing rapid changes to ensure the people are given the most effective method of vector control. Lastly, antimalarial drugs can also be used to prevent infection from developing into a clinical disease. However, there has also been an increase resistance to antimalarial medicine.[61] In 2015 the World Health Organization (WHO) drew up a plan to address vivax malaria.[62]
Treatment


Chloroquine remains the treatment of choice for vivax malaria,[63] except in Indonesia's Irian Jaya (Western New Guinea) region and the geographically contiguous Papua New Guinea, where chloroquine resistance is common (up to 20% resistance). Chloroquine resistance is an increasing problem in other parts of the world, such as Korea[64] and India.
When chloroquine resistance is common or when chloroquine is contraindicated, then artesunate is the drug of choice, except in the U.S., where it is not approved for use.[65] Where an artemisinin-based combination therapy has been adopted as the first-line treatment for P. falciparum malaria, it may also be used for P. vivax malaria in combination with primaquine for radical cure.[63] An exception is artesunate plus sulfadoxine-pyrimethamine (AS+SP), which is not effective against P. vivax in many places.[63] Mefloquine is a good alternative and in some countries is more readily available.[66] Atovaquone-proguanil is an effective alternative in patients unable to tolerate chloroquine.[67] Quinine may be used to treat vivax malaria but is associated with inferior outcomes.[68]
32–100% of patients will relapse following successful treatment of P. vivax infection if a radical cure (inactivation of liver stages) is not given.[69][70][71] Eradication of the liver stages is achieved by giving primaquine but patients with glucose-6-phosphate dehydrogenase deficiency are at risk for haemolysis.[72] G6PD-testing is therefore very important, both in endemic areas and in travelers.[73] At least a 14-day course of primaquine is required for the radical treatment of P. vivax malaria.[63] The idea that primaquine kills parasites in the liver is the traditional assumption. However, it has been suggested that primaquine might, to a currently unknown extent, also inactivate noncirculating, extrahepatic merozoite.[74]
Mass-treating populations with primaquine can kill the hypnozoites, exempting those individuals with G6PD deficiency, however, the standard regimen requires a daily pill for 14 days.[10]
Epidemiology
_species_by_country_of_origin_for_imported_cases_to_non-endemic_countries.png.webp)
Plasmodium vivax is found mainly in Asia, Latin America, and in some parts of Africa.[76][77] P. vivax is believed to have originated in Asia, but recent studies have shown that wild chimpanzees and gorillas throughout central Africa are endemically infected with parasites that are closely related to human P. vivax. These findings indicate that human P. vivax is of African origin.[78] Plasmodium vivax accounts for 65% of malaria cases in Asia and South America.[50] Unlike Plasmodium falciparum, Plasmodium vivax is capable of undergoing sporogonic development[79] in the mosquito at lower temperatures.[80] It has been estimated that 2.5 billion people are at risk of infection with this organism.[81]
Although the Americas contribute 22% of the global area at risk, high endemic areas are generally sparsely populated and the region contributes only 6% to the total population at risk. In Africa, the widespread lack of the Duffy antigen in the population has ensured that stable transmission is constrained to Madagascar and parts of the Horn of Africa. It contributes 3.5% of global population at risk. Central Asia is responsible for 82% of global population at risk with high endemic areas coinciding with dense populations particularly in India and Myanmar. South East Asia has areas of high endemicity in Indonesia and Papua New Guinea and overall contributes 9% of global population at risk.[82]P. vivax is carried by at least 71 mosquito species. Many vivax vectors thrive in temperate climates—as far north as Finland. Some prefer to bite outdoors or during the daytime, hampering the effectiveness of indoor insecticide and bed nets. Several key vector species have yet to be grown in the lab for closer study, and insecticide resistance is unquantified.[50]
Korea
P. vivax is the only indigenous malaria parasite on the Korean peninsula. In the years following the Korean War (1950–53), malaria-eradication campaigns successfully reduced the number of new cases of the disease in North Korea and South Korea. In 1979, World Health Organization declared the Korean peninsula vivax malaria-free, but the disease unexpectedly re-emerged in the late 1990s and still persists today. Several factors contributed to the re-emergence of the disease, including reduced emphasis on malaria control after 1979, floods and famine in North Korea, emergence of drug resistance and possibly global warming. Most cases are identified along the Korean Demilitarized Zone. As such, vivax malaria offers the two Koreas an opportunity to work together on an important health problem that affects both countries.[83][84]
Research
In 2013 a Phase IIb trial was completed that studied a single-dose alternative drug named tafenoquine.[85] It is an 8-aminoquinoline, of the same family as primaquine,[86] developed by researchers at the Walter Reed Army Institute of Research in the 1970s and tested in safety trials. It languished, however, until the push for malaria elimination sparked new interest in primaquine alternatives.[50]
Among patients who received a 600-mg dose, 91% were relapse-free after 6 months. Among patients who received primaquine, 24% relapsed within 6 months. "The data are absolutely spectacular," Wells says. Ideally, he says, researchers will be able to combine the safety data from the Army's earlier trials with the new study in a submission to the U.S. Food and Drug Administration for approval. Like primaquine, tafenoquine causes hemolysis in people who are G6PD deficient.In 2013 researchers produced cultured human "microlivers" that supported liver stages of both P. falciparum and P. vivax and may have also created hypnozoites.[50][87][50]
History

P. vivax was used between 1917 and the 1940s for malariotherapy, that is, to create very high fevers to combat certain diseases such as tertiary syphilis. In 1917, the inventor of this technique, Julius Wagner-Jauregg, received the Nobel Prize in Physiology or Medicine for his discoveries.[88][89][90]
However, the technique was dangerous, killing about 15% of patients, so it is no longer in use.[91]
Drug Targets
Given that drugs that target the various life stages of the parasite can sometimes have undesirable side effects, it is desirable to come up with drug molecules targeting specific proteins/enzymes that are essential for the parasite's survival or that can compromise the fitness of the organism. Enzymes in the Purine salvage pathway had been favorite targets to this end.[92]
See also
References
- ↑ Coatney, G. Robert; Collins, William E.; Warren, McWilson; Contacos, Peter George (1971). "Plasmodium vivax (Grassi and Feletti, 1890)". The primate malarias. Division of Parasitic Disease, CDC. pp. 43–44. LCCN 71-610655. Archived from the original on 2019-08-26. Retrieved 2023-03-21.
- 1 2 Adams, JH; Mueller, I (1 September 2017). "The Biology of Plasmodium vivax". Cold Spring Harbor Perspectives in Medicine. 7 (9): a025585. doi:10.1101/cshperspect.a025585. PMC 5580510. PMID 28490540.
- ↑ "Plasmodium vivax malaria (Concept Id: C0024537) - MedGen - NCBI". www.ncbi.nlm.nih.gov. Archived from the original on 22 December 2022. Retrieved 17 June 2023.
- ↑ White, N. J. (15 January 2008). "Plasmodium knowlesi: The Fifth Human Malaria Parasite". Clinical Infectious Diseases. 46 (2): 172–173. doi:10.1086/524889. PMID 18171246.
- ↑ Baird, J. Kevin (November 2007). "Neglect of Plasmodium vivax malaria". Trends in Parasitology. 23 (11): 533–539. doi:10.1016/j.pt.2007.08.011. PMID 17933585. Archived from the original on 2022-05-11. Retrieved 2023-03-21.
- ↑ Anstey, Nicholas M.; Douglas, Nicholas M.; Poespoprodjo, Jeanne R.; Price, Ric N. (2012). Plasmodium vivax. Advances in Parasitology. Vol. 80. pp. 151–201. doi:10.1016/b978-0-12-397900-1.00003-7. ISBN 978-0-12-397900-1. PMID 23199488.
- ↑ Crompton, Peter D.; Moebius, Jacqueline; Portugal, Silvia; Waisberg, Michael; Hart, Geoffrey; Garver, Lindsey S.; Miller, Louis H.; Barillas-Mury, Carolina; Pierce, Susan K. (21 March 2014). "Malaria Immunity in Man and Mosquito: Insights into Unsolved Mysteries of a Deadly Infectious Disease". Annual Review of Immunology. 32 (1): 157–187. doi:10.1146/annurev-immunol-032713-120220. PMC 4075043. PMID 24655294.
- ↑ Charon, Justine; Grigg, Matthew J.; Eden, John-Sebastian; Piera, Kim A.; Rana, Hafsa; William, Timothy; Rose, Karrie; Davenport, Miles P.; Anstey, Nicholas M.; Holmes, Edward C. (30 December 2019). "Novel RNA viruses associated with Plasmodium vivax in human malaria and Leucocytozoon parasites in avian disease". PLOS Pathogens. 15 (12): e1008216. doi:10.1371/journal.ppat.1008216. PMC 6953888. PMID 31887217.
- ↑ Milner DA, Jr (2 January 2018). "Malaria Pathogenesis". Cold Spring Harbor Perspectives in Medicine. 8 (1): a025569. doi:10.1101/cshperspect.a025569. PMC 5749143. PMID 28533315.
- 1 2 Flannery, Erika L.; Markus, Miles B.; Vaughan, Ashley M. (July 2019). "Plasmodium vivax". Trends in Parasitology. 35 (7): 583–584. doi:10.1016/j.pt.2019.04.005. PMID 31176582. S2CID 182948097. Archived from the original on 1 July 2023. Retrieved 3 June 2023.
- 1 2 3 4 5 Menkin-Smith, Lacey; Winders, Walter T. (2023). "Plasmodium vivax Malaria". StatPearls. StatPearls Publishing. PMID 30855917. Archived from the original on 22 December 2022. Retrieved 6 June 2023.
- ↑ Campbell, Neil A.; Reece, Jane B. (2002). Biology (6th ed.). Pearson Education. pp. 540–541. ISBN 978-0-201-75054-6.
- 1 2 Bryant, Bronwen Jean; Knights, Kathleen Mary (2011). Pharmacology for Health Professionals. Elsevier Australia. p. 893. ISBN 978-0-7295-3929-6. Archived from the original on 2023-07-01. Retrieved 2023-06-15.
- ↑ McKenzie, F. Ellis; Jeffery, Geoffrey M.; Collins, William E. (June 2002). "PLASMODIUM VIVAX BLOOD-STAGE DYNAMICS". Journal of Parasitology. 88 (3): 521–535. doi:10.1645/0022-3395(2002)088[0521:PVBSD]2.0.CO;2. Archived from the original on 20 May 2016. Retrieved 17 June 2023.
- ↑ Venugopal, K; Hentzschel, F; Valkiūnas, G; Marti, M (March 2020). "Plasmodium asexual growth and sexual development in the haematopoietic niche of the host". Nature Reviews. Microbiology. 18 (3): 177–189. doi:10.1038/s41579-019-0306-2. PMC 7223625. PMID 31919479.
- ↑ The Epidemiology of Plasmodium vivax: History, Hiatus and Hubris, Part B. Academic Press. 31 January 2013. p. 111. ISBN 978-0-12-407877-2. Archived from the original on 1 July 2023. Retrieved 22 June 2023.
- ↑ Sato, Shigeharu (7 January 2021). "Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology". Journal of Physiological Anthropology. 40 (1). doi:10.1186/s40101-020-00251-9. Archived from the original on 29 December 2022. Retrieved 22 June 2023.
- ↑ Chavatte JM, Tan SB, Snounou G, Lin RT (2015). "Molecular characterization of misidentified Plasmodium ovale imported cases in Singapore". Malar J. 14: 454. doi:10.1186/s12936-015-0985-8. PMC 4650842. PMID 26577930.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Ménard, R; Tavares, J; Cockburn, I; Markus, M; Zavala, F; Amino, R (2013). "Looking under the skin: the first steps in malarial infection and immunity". Nature Reviews Microbiology. 11 (10): 701–712. doi:10.1038/nrmicro3111. PMID 24037451. S2CID 21437365.
- ↑ "Malaria factsheet". GOV.UK. Archived from the original on 1 September 2022. Retrieved 4 June 2023.
- ↑ White, Nicholas J. (11 October 2011). "Determinants of relapse periodicity in Plasmodium vivax malaria". Malaria Journal. 10: 297. doi:10.1186/1475-2875-10-297. ISSN 1475-2875. PMC 3228849. PMID 21989376.
- ↑ Schäfer, Carola; Zanghi, Gigliola; Vaughan, Ashley M.; Kappe, Stefan H. I. (8 October 2021). "Plasmodium vivax Latent Liver Stage Infection and Relapse: Biological Insights and New Experimental Tools". Annual Review of Microbiology. 75: 87–106. doi:10.1146/annurev-micro-032421-061155. ISSN 1545-3251. PMID 34196569. S2CID 235696976. Archived from the original on 1 July 2021. Retrieved 5 June 2023.
- ↑ White, Nicholas J. (December 2016). "Why Do Some Primate Malarias Relapse?". Trends in Parasitology. 32 (12): 918–920. doi:10.1016/j.pt.2016.08.014. PMC 5134685. PMID 27743866.
- ↑ Markus, Miles B. (November 2018). "Biological concepts in recurrent Plasmodium vivax malaria". Parasitology. 145 (13): 1765–1771. doi:10.1017/S003118201800032X. PMID 29564998. S2CID 206250162.
- ↑ Markus, Miles B. (July 2017). "Malaria Eradication and the Hidden Parasite Reservoir". Trends in Parasitology. 33 (7): 492–495. doi:10.1016/j.pt.2017.03.002. PMID 28366603.
- ↑ Markus, Miles B. (2022). "Theoretical origin of genetically homologous Plasmodium vivax malarial recurrences". Southern African Journal of Infectious Diseases. 37 (1): 369. doi:10.4102/sajid.v37i1.369. PMC 8991251. PMID 35399558.
- ↑ Van den Enden J. "Illustrated Lecture Notes on Tropical Medicine". Archived from the original on 2015-11-23. Retrieved 2015-11-01.
- ↑ Motta, Francis C.; McGoff, Kevin; Moseley, Robert C.; Cho, Chun-Yi; Kelliher, Christina M.; Smith, Lauren M.; Ortiz, Michael S.; Leman, Adam R.; Campione, Sophia A.; Devos, Nicolas; Chaorattanakawee, Suwanna; Uthaimongkol, Nichaphat; Kuntawunginn, Worachet; Thongpiam, Chadin; Thamnurak, Chatchadaporn; Arsanok, Montri; Wojnarski, Mariusz; Vanchayangkul, Pattaraporn; Boonyalai, Nonlawat; Smith, Philip L.; Spring, Michele D.; Jongsakul, Krisada; Chuang, Ilin; Harer, John; Haase, Steven B. (13 June 2023). "The parasite intraerythrocytic cycle and human circadian cycle are coupled during malaria infection". Proceedings of the National Academy of Sciences of the United States of America. 120 (24): e2216522120. doi:10.1073/pnas.2216522120. ISSN 1091-6490. PMID 37279274. Archived from the original on 1 July 2023. Retrieved 8 June 2023.
- ↑ Mehlhorn, Heinz (2016). "Schüffner's Dots". Encyclopedia of Parasitology. Springer. pp. 2489–2490. doi:10.1007/978-3-662-43978-4_2816. ISBN 978-3-662-43978-4. Archived from the original on 14 June 2018. Retrieved 14 June 2023.
- 1 2 Prevention, CDC-Centers for Disease Control and (22 March 2022). "CDC - Malaria - About Malaria - Disease". www.cdc.gov. Archived from the original on 24 May 2023. Retrieved 5 June 2023.
- ↑ Aly, AS; Vaughan, AM; Kappe, SH (2009). "Malaria parasite development in the mosquito and infection of the mammalian host". Annual Review of Microbiology. 63: 195–221. doi:10.1146/annurev.micro.091208.073403. PMC 2841446. PMID 19575563.
- ↑ Bennink, S; Kiesow, MJ; Pradel, G (July 2016). "The development of malaria parasites in the mosquito midgut". Cellular Microbiology. 18 (7): 905–18. doi:10.1111/cmi.12604. PMC 5089571. PMID 27111866.
- ↑ Liu, Zhenyu; Miao, Jun; Cui, Liwang (November 2011). "Gametocytogenesis in malaria parasite: commitment, development and regulation". Future Microbiology. 6 (11): 1351–1369. doi:10.2217/fmb.11.108. Archived from the original on 1 July 2023. Retrieved 21 June 2023.
- ↑ Tembhare, Prashant; Shirke, Shalaka; Subramanian, P. G.; Sehgal, Kunal; Gujral, Sumeet (1 April 2009). "Exflagellated microgametes of Plasmodium vivax in human peripheral blood: A case report and review of the literature". Indian Journal of Pathology and Microbiology. 52 (2): 252. doi:10.4103/0377-4929.48936. ISSN 0377-4929. Archived from the original on 6 February 2023. Retrieved 23 June 2023.
- ↑ Dash, Manoswini; Sachdeva, Sherry; Bansal, Abhisheka; Sinha, Abhinav (15 June 2022). "Gametogenesis in Plasmodium: Delving Deeper to Connect the Dots". Frontiers in Cellular and Infection Microbiology. 12. doi:10.3389/fcimb.2022.877907. Archived from the original on 1 July 2023. Retrieved 24 June 2023.
- ↑ Crutcher, James M.; Hoffman, Stephen L. (1996). "Malaria". Medical Microbiology (4th ed.). University of Texas Medical Branch at Galveston. ISBN 978-0-9631172-1-2. Archived from the original on 2023-03-19. Retrieved 8 June 2023.
- ↑ Keleta, Yacob; Ramelow, Julian; Cui, Liwang; Li, Jun (29 November 2021). "Molecular interactions between parasite and mosquito during midgut invasion as targets to block malaria transmission". NPJ Vaccines. 6 (1): 140. doi:10.1038/s41541-021-00401-9. ISSN 2059-0105. PMC 8630063. PMID 34845210.
- ↑ Sinden, RE (April 2015). "The cell biology of malaria infection of mosquito: advances and opportunities". Cellular Microbiology. 17 (4): 451–66. doi:10.1111/cmi.12413. PMC 4409862. PMID 25557077.
- ↑ Henshaw, Jonathan M.; Jones, Adam G.; Schärer, Lukas (6 July 2022). "Anisogamy explains why males benefit more from additional matings". Nature Communications. 13 (1): 3893. doi:10.1038/s41467-022-31620-w. ISSN 2041-1723. Archived from the original on 1 July 2023. Retrieved 25 June 2023.
- ↑ Steinbuechel, M; Matuschewski, K (February 2009). "Role for the Plasmodium sporozoite-specific transmembrane protein S6 in parasite motility and efficient malaria transmission". Cellular microbiology. 11 (2): 279–88. doi:10.1111/j.1462-5822.2008.01252.x. PMID 19016774. Archived from the original on 1 July 2023. Retrieved 25 June 2023.
- ↑ Zollner, Gabriela E.; Ponsa, Narong; Garman, Gabriel W.; Poudel, Shreekanta; Bell, Jeffrey A.; Sattabongkot, Jetsumon; Coleman, Russell E.; Vaughan, Jefferson A. (3 August 2006). "Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes". Malaria Journal. 5: 68. doi:10.1186/1475-2875-5-68. ISSN 1475-2875. PMC 1557861. PMID 16887043.
- ↑ Balabaskaran Nina, Praveen; Mohanty, Ajeet Kumar; Ballav, Shuvankar; Vernekar, Smita; Bhinge, Sushma; D’souza, Maria; Walke, Jayashree; Manoharan, Suresh Kumar; Mascarenhas, Anjali; Gomes, Edwin; Chery, Laura; Valecha, Neena; Kumar, Ashwani; Rathod, Pradipsinh K. (December 2017). "Dynamics of Plasmodium vivax sporogony in wild Anopheles stephensi in a malaria-endemic region of Western India". Malaria Journal. 16 (1): 284. doi:10.1186/s12936-017-1931-8. PMC 5504555. PMID 28693607.
- ↑ Jordan, E.L. (2001). Invertebrate Zoology (Multicolour Edition). S. Chand Publishing. p. 187. ISBN 978-81-219-0367-7. Archived from the original on 1 July 2023. Retrieved 18 June 2023.
- ↑ Li, J.; Collins, W. E.; Wirtz, R. A.; Rathore, D.; Lal, A.; McCutchan, T. F. (2001). "Geographic subdivision of the range of the malaria parasite Plasmodium vivax". Emerging Infectious Diseases. 7 (1): 35–42. doi:10.3201/eid0701.010105. PMC 2631686. PMID 11266292.
- ↑ "Taxonomy browser (Plasmodium vivax)". www.ncbi.nlm.nih.gov. Archived from the original on 1 July 2023. Retrieved 8 June 2023.
- ↑ "Plasmodium vivax". pubchem.ncbi.nlm.nih.gov. Archived from the original on 1 July 2023. Retrieved 14 June 2023.
- ↑ de Oliveira, Thaís C.; Rodrigues, Priscila T.; Early, Angela M.; Duarte, Ana Maria R. C.; Buery, Julyana C.; Bueno, Marina G.; Catão-Dias, José L.; Cerutti, Crispim; Rona, Luísa D. P.; Neafsey, Daniel E.; Ferreira, Marcelo U. (1 December 2021). "Plasmodium simium: Population Genomics Reveals the Origin of a Reverse Zoonosis". The Journal of Infectious Diseases. 224 (11): 1950–1961. doi:10.1093/infdis/jiab214. ISSN 1537-6613. Archived from the original on 29 January 2022. Retrieved 21 June 2023.
- ↑ Emerging Infectious Diseases. National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). 2001. p. 41. Archived from the original on 1 July 2023. Retrieved 21 June 2023.
- ↑ Bar, Daniel (16 February 2011). "Evidence of Massive Horizontal Gene Transfer Between Humans and Plasmodium vivax". Nature Precedings. doi:10.1038/npre.2011.5690.1.
- 1 2 3 4 5 6 7 8 Vogel, G. (8 November 2013). "The Forgotten Malaria". Science. 342 (6159): 684–687. Bibcode:2013Sci...342..684V. doi:10.1126/science.342.6159.684. PMID 24202156.
- ↑ Guadarrama-Conzuelo, Francisco; Saad Manzanera, Assad D (1 September 2019). "Singultus as an Unusual Debut of Plasmodium vivax Malaria". Cureus. 11 (9): e5548. doi:10.7759/cureus.5548. PMC 6820320. PMID 31695971.
- ↑ Mohapatra, MK; Padhiary, KN; Mishra, DP; Sethy, G (March 2002). "Atypical manifestations of Plasmodium vivax malaria". Indian Journal of Malariology. 39 (1–2): 18–25. PMID 14686106.
- ↑ Markus, Miles B. (November 2011). "Malaria: Origin of the Term 'Hypnozoite'". Journal of the History of Biology. 44 (4): 781–786. doi:10.1007/s10739-010-9239-3. PMID 20665090. S2CID 1727294.
- ↑ Heymann, David L, ed. (2015). Control of Communicable Diseases Manual. American Public Health Association. doi:10.2105/CCDM.2745. ISBN 978-0-87553-274-5. Archived from the original on 2022-10-10. Retrieved 2023-03-21.
- ↑ Nadjm, B; Behrens, RH (June 2012). "Malaria: an update for physicians". Infectious Disease Clinics of North America. 26 (2): 243–59. doi:10.1016/j.idc.2012.03.010. PMID 22632637. Archived from the original on 2020-09-22.
- ↑ Manguin S, Foumane V, Besnard P, Fortes F, Carnevale P (July 2017). "Malaria overdiagnosis and subsequent overconsumption of antimalarial drugs in Angola: Consequences and effects on human health". Acta Tropica. 171: 58–63. doi:10.1016/j.actatropica.2017.03.022. PMID 28356231.
- ↑ Orish VN, Ansong JY, Onyeabor OS, Sanyaolu AO, Oyibo WA, Iriemenam NC (October 2016). "Overdiagnosis and overtreatment of malaria in children in a secondary healthcare centre in Sekondi-Takoradi, Ghana". Tropical Doctor. 46 (4): 191–198. doi:10.1177/0049475515622861. PMID 26738767. S2CID 27793830. Archived from the original on 2021-08-28. Retrieved 2020-08-03.
- ↑ Yegorov S, Galiwango RM, Ssemaganda A, Muwanga M, Wesonga I, Miiro G, et al. (November 2016). "Low prevalence of laboratory-confirmed malaria in clinically diagnosed adult women from the Wakiso district of Uganda". Malaria Journal. 15 (1): 555. doi:10.1186/s12936-016-1604-z. PMC 5109652. PMID 27842555.
- ↑ Chu, CS; White, NJ (April 2021). "The prevention and treatment of Plasmodium vivax malaria". PLOS Medicine. 18 (4): e1003561. doi:10.1371/journal.pmed.1003561. PMC 8064578. PMID 33891587.
- ↑ Abba, Katharine; Kirkham, Amanda J; Olliaro, Piero L; Deeks, Jonathan J; Donegan, Sarah; Garner, Paul; Takwoingi, Yemisi (18 December 2014). "Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium vivax malaria in endemic countries". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD011431. Archived from the original on 25 September 2022. Retrieved 20 June 2023.
- ↑ "Malaria Fact Sheet". World Health Organization. Archived from the original on 3 September 2014. Retrieved 20 October 2016.
- ↑ Control and elimination of Plasmodium vivax malaria – A technical brief. WHO. July 2015. ISBN 978-92-4-150924-4. Archived from the original on September 12, 2015.
- 1 2 3 4 Organization, World Health (April 2015). Guidelines for the treatment of malaria. ISBN 978-92-4-154792-5. Archived from the original on April 25, 2015.
- ↑ Kim, Tae Hyong; Yeom, Joon-Sup; Lee, Kkot Sil; Kim, Eu Suk; Park, Jae-Won; Jun, Gyo; Lim, Hyeong-Seok (1 February 2009). "Chloroquine-resistant Plasmodium vivax in the Republic of Korea". The American Journal of Tropical Medicine and Hygiene. 80 (2): 215–217. doi:10.4269/ajtmh.2009.80.215. PMID 19190216.
- ↑ Pukrittayakamee, Sasithon; Chantra, Arun; Simpson, Julie A.; Vanijanonta, Sirivan; Clemens, Ralf; Looareesuwan, Sornchai; White, Nicholas J. (1 June 2000). "Therapeutic Responses to Different Antimalarial Drugs in Vivax Malaria". Antimicrobial Agents and Chemotherapy. 44 (6): 1680–1685. doi:10.1128/aac.44.6.1680-1685.2000. PMC 89932. PMID 10817728.
- ↑ Maguire, J. D.; Marwoto, H.; Richie, T. L.; Fryauff, D. J.; Baird, J. K.; Baird, JK (15 April 2006). "Mefloquine Is Highly Efficacious against Chloroquine-Resistant Plasmodium vivax Malaria and Plasmodium falciparum Malaria in Papua, Indonesia". Clinical Infectious Diseases. 42 (8): 1067–1072. doi:10.1086/501357. PMID 16575721.
- ↑ Looareesuwan, S.; Wilairatana, P.; Glanarongran, R.; Indravijit, K.A.; Supeeranontha, L.; Chinnapha, S.; Scott, T.R.; Chulay, J.D. (November 1999). "Atovaquone and proguanil hydrochloride followed by primaquine for treatment of Plasmodium vivax malaria in Thailand". Transactions of the Royal Society of Tropical Medicine and Hygiene. 93 (6): 637–640. doi:10.1016/s0035-9203(99)90079-2. PMID 10717754.
- ↑ Achan, Jane; Talisuna, Ambrose O; Erhart, Annette; Yeka, Adoke; Tibenderana, James K; Baliraine, Frederick N; Rosenthal, Philip J; D'Alessandro, Umberto (December 2011). "Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria". Malaria Journal. 10 (1): 144. doi:10.1186/1475-2875-10-144. PMC 3121651. PMID 21609473.
- ↑ Wiselogle FY (1943). J.W. Edwards (ed.). A survey of antimalarial drugs, 1941–1945 (2 vols.). Ann Arbor, Michigan.
- ↑ Hankey, Daniel D.; Donovan, William N.; Jones, Ralph; Coker, Walter G.; Coatney, G. Robert; Alving, Alf S.; Garrison, Paul L. (1 November 1953). "Korean Vivax Malaria: II. Curative Treatment with Pamaquine and Primaquine". The American Journal of Tropical Medicine and Hygiene. 2 (6): 970–976. doi:10.4269/ajtmh.1953.2.970. PMID 13104805.
- ↑ Orlov, V S; Adak, T; Sharma, V P (1 July 1998). "Studies on the Plasmodium vivax relapse pattern in Delhi, India". The American Journal of Tropical Medicine and Hygiene. 59 (1): 175–179. doi:10.4269/ajtmh.1998.59.175. PMID 9684649.
- ↑ Baird, J. K.; Hoffman, S. L. (1 November 2004). "Primaquine Therapy for Malaria". Clinical Infectious Diseases. 39 (9): 1336–1345. doi:10.1086/424663. PMID 15494911.
- ↑ Saleri, Nuccia; Gulletta, Maurizio; Matteelli, Alberto; Caligaris, Silvio; Tomasoni, Lina Rachele; Antonini, Benvenuto; Perandin, Francesca; Castelli, Francesco (1 March 2006). "Acute Respiratory Distress Syndrome in Plasmodium vivax Malaria in Traveler Returning From Venezuela". Journal of Travel Medicine. 13 (2): 112–113. doi:10.1111/j.1708-8305.2006.00024.x. PMID 16553597.
- ↑ Markus, MB (2022). "How does primaquine prevent Plasmodium vivax malarial recurrences?". Trends in Parasitology. 38 (11): 924–925. doi:10.1016/j.pt.2022.09.006. PMID 36180306. S2CID 252579139.
- ↑ Tatem AJ, Jia P, Ordanovich D, Falkner M, Huang Z, Howes R; et al. (2017). "The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics". Lancet Infect Dis. 17 (1): 98–107. doi:10.1016/S1473-3099(16)30326-7. PMC 5392593. PMID 27777030.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "Biology: Malaria Parasites". Malaria. CDC. 2004-04-23. Archived from the original on 2008-10-13. Retrieved 2008-09-30.
- ↑ Lindsay, Sw; Hutchinson, Ra (September 2006). "Malaria and deaths in the English marshes – Authors' reply". The Lancet. 368 (9542): 1152. doi:10.1016/S0140-6736(06)69467-1. S2CID 53296779.
- ↑ Liu, Weimin; Li, Yingying; Shaw, Katharina S.; Learn, Gerald H.; Plenderleith, Lindsey J.; Malenke, Jordan A.; Sundararaman, Sesh A.; Ramirez, Miguel A.; Crystal, Patricia A.; Smith, Andrew G.; Bibollet-Ruche, Frederic; Ayouba, Ahidjo; Locatelli, Sabrina; Esteban, Amandine; Mouacha, Fatima; Guichet, Emilande; Butel, Christelle; Ahuka-Mundeke, Steve; Inogwabini, Bila-Isia; Ndjango, Jean-Bosco N.; Speede, Sheri; Sanz, Crickette M.; Morgan, David B.; Gonder, Mary K.; Kranzusch, Philip J.; Walsh, Peter D.; Georgiev, Alexander V.; Muller, Martin N.; Piel, Alex K.; Stewart, Fiona A.; Wilson, Michael L.; Pusey, Anne E.; Cui, Liwang; Wang, Zenglei; Färnert, Anna; Sutherland, Colin J.; Nolder, Debbie; Hart, John A.; Hart, Terese B.; Bertolani, Paco; Gillis, Amethyst; LeBreton, Matthew; Tafon, Babila; Kiyang, John; Djoko, Cyrille F.; Schneider, Bradley S.; Wolfe, Nathan D.; Mpoudi-Ngole, Eitel; Delaporte, Eric; Carter, Richard; Culleton, Richard L.; Shaw, George M.; Rayner, Julian C.; Peeters, Martine; Hahn, Beatrice H.; Sharp, Paul M. (May 2014). "African origin of the malaria parasite Plasmodium vivax". Nature Communications. 5 (1): 3346. Bibcode:2014NatCo...5.3346L. doi:10.1038/ncomms4346. PMC 4089193. PMID 24557500.
- ↑ "sporogonic". The Free Dictionary. Archived from the original on 2022-10-04. Retrieved 2023-03-21.
- ↑ Gething, Peter W; Van Boeckel, Thomas P; Smith, David L; Guerra, Carlos A; Patil, Anand P; Snow, Robert W; Hay, Simon I (December 2011). "Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax". Parasites & Vectors. 4 (1): 92. doi:10.1186/1756-3305-4-92. PMC 3115897. PMID 21615906.
- ↑ Gething, Peter W.; Elyazar, Iqbal R. F.; Moyes, Catherine L.; Smith, David L.; Battle, Katherine E.; Guerra, Carlos A.; Patil, Anand P.; Tatem, Andrew J.; Howes, Rosalind E.; Myers, Monica F.; George, Dylan B.; Horby, Peter; Wertheim, Heiman F. L.; Price, Ric N.; Müeller, Ivo; Baird, J. Kevin; Hay, Simon I. (6 September 2012). "A Long Neglected World Malaria Map: Plasmodium vivax Endemicity in 2010". PLOS Neglected Tropical Diseases. 6 (9): e1814. doi:10.1371/journal.pntd.0001814. PMC 3435256. PMID 22970336.
- ↑ Battle, Katherine E.; Gething, Peter W.; Elyazar, Iqbal R.F.; Moyes, Catherine L.; Sinka, Marianne E.; Howes, Rosalind E.; Guerra, Carlos A.; Price, Ric N.; Baird, J. Kevin; Hay, Simon I. (2012). The Global Public Health Significance of Plasmodium vivax. Advances in Parasitology. Vol. 80. pp. 1–111. doi:10.1016/b978-0-12-397900-1.00001-3. ISBN 978-0-12-397900-1. PMID 23199486. Archived from the original on 2022-05-18. Retrieved 2023-03-21.
- ↑ Konings, Frank (19 May 2008). "For Re-Eradication of Malaria in Korea". The Korea Times. Archived from the original on 19 May 2008. Retrieved 21 March 2023.
- ↑ Konings, Frank (9 July 2008). "The Korean War Against Malaria". Far Eastern Economic Review. 171 (6). Archived from the original on 27 August 2008.
- ↑ Llanos-Cuentas, Alejandro; Lacerda, Marcus V; Rueangweerayut, Ronnatrai; Krudsood, Srivicha; Gupta, Sandeep K; Kochar, Sanjay K; Arthur, Preetam; Chuenchom, Nuttagarn; Möhrle, Jörg J; Duparc, Stephan; Ugwuegbulam, Cletus; Kleim, Jörg-Peter; Carter, Nick; Green, Justin A; Kellam, Lynda (March 2014). "Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study". The Lancet. 383 (9922): 1049–1058. doi:10.1016/S0140-6736(13)62568-4. PMID 24360369. S2CID 205971592.
- ↑ "Tafenoquine". MMV. Archived from the original on 2014-02-21. Retrieved 2014-02-17.
- ↑ Habtamu, Kassahun; Petros, Beyene; Yan, Guiyun (15 December 2022). "Plasmodium vivax: the potential obstacles it presents to malaria elimination and eradication". Tropical Diseases, Travel Medicine and Vaccines. 8 (1): 27. doi:10.1186/s40794-022-00185-3. ISSN 2055-0936. Archived from the original on 23 December 2022. Retrieved 16 June 2023.
- ↑ "The Nobel Prize in Physiology or Medicine 1927". Nobelprize.org. Nobel Media AB. Archived from the original on 26 September 2006. Retrieved 20 March 2014.
- ↑ Kohl, F. (July 1993). "[Wagner von Jauregg and development of malaria therapy]". Psychiatrische Praxis. 20 (4): 157–159. ISSN 0303-4259. Archived from the original on 2 September 2022. Retrieved 21 June 2023.
- ↑ Raju, T. N. (21 November 1998). "The Nobel chronicles. 1927: Julius Wagner-Jauregg (1857-1940)". Lancet (London, England). 352 (9141): 1714. doi:10.1016/s0140-6736(05)61500-0. ISSN 0140-6736. Archived from the original on 25 March 2023. Retrieved 21 June 2023.
- ↑ Vogel, G. (8 November 2013). "Malaria as Lifesaving Therapy". Science. 342 (6159): 686. Bibcode:2013Sci...342..686V. doi:10.1126/science.342.6159.686. PMID 24202157.
- ↑ Chu, CS; White, NJ (April 2021). "The prevention and treatment of Plasmodium vivax malaria". PLOS Medicine. 18 (4): e1003561. doi:10.1371/journal.pmed.1003561. PMC 8064578. PMID 33891587.