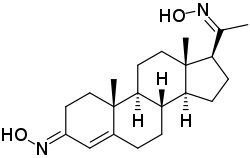
Progesterone dioxime
 | |
| Clinical data | |
|---|---|
| Other names | Progesterone 3,20-dioxime; P4-3,20-DO; Pregn-4-ene-3,20-dione 3,20-dioxime; 3,20-Di(hydroxyimino)pregn-4-en-3-one |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H32N2O2 |
| Molar mass | 344.499 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 238–242 °C (460–468 °F) (recrystallized from alcohol) |
SMILES
| |
InChI
| |
Progesterone dioxime, or progesterone 3,20-dioxime (P4-3,20-DO), also known as 3,20-di(hydroxyimino)pregn-4-en-3-one, is a progesterone derivative which was never marketed.[1] It is a progestogen oxime – specifically, the C3 and C20 dioxime of the progestogen progesterone.[1] Progesterone C3 and C20 oxime conjugates have been found to be water-soluble prodrugs of progesterone and pregnane neurosteroids.[2][3][4][5]
See also
References
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1024–. ISBN 978-1-4757-2085-3.
- ↑ MacNevin CJ, Atif F, Sayeed I, Stein DG, Liotta DC (October 2009). "Development and screening of water-soluble analogues of progesterone and allopregnanolone in models of brain injury". J. Med. Chem. 52 (19): 6012–23. doi:10.1021/jm900712n. PMID 19791804.
- ↑ Guthrie DB, Stein DG, Liotta DC, Lockwood MA, Sayeed I, Atif F, Arrendale RF, Reddy GP, Evers TJ, Marengo JR, Howard RB, Culver DG, Natchus MG (May 2012). "Water-soluble progesterone analogues are effective, injectable treatments in animal models of traumatic brain injury". ACS Med Chem Lett. 3 (5): 362–6. doi:10.1021/ml200303r. PMC 4025794. PMID 24900479.
- ↑ Wali B, Sayeed I, Guthrie DB, Natchus MG, Turan N, Liotta DC, Stein DG (October 2016). "Evaluating the neurotherapeutic potential of a water-soluble progesterone analog after traumatic brain injury in rats". Neuropharmacology. 109: 148–158. doi:10.1016/j.neuropharm.2016.05.017. PMID 27267687. S2CID 19906601.
- ↑ Basu, Krishnakali; Mitra, Ashim K. (1990). "Effects of 3-hydrazone modification on the metabolism and protein binding of progesterone". International Journal of Pharmaceutics. 65 (1–2): 109–114. doi:10.1016/0378-5173(90)90015-V. ISSN 0378-5173.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.