Treponema pallidum
| Treponema pallidum | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Spirochaetota |
| Class: | Spirochaetia |
| Order: | Spirochaetales |
| Family: | Treponemataceae |
| Genus: | Treponema |
| Species: | T. pallidum |
| Binomial name | |
| Treponema pallidum Schaudinn & Hoffmann, 1905 | |
Treponema pallidum, formerly known as Spirochaeta pallida, is a spirochaete bacterium with various subspecies that cause the diseases syphilis, bejel (also known as endemic syphilis), and yaws. It is transmitted only among humans.[1] It is a helically coiled microorganism usually 6–15 μm long and 0.1–0.2 μm wide.[2] T. pallidum's lack of either a tricarboxylic acid cycle or oxidative phosphorylation results in minimal metabolic activity.[3] The treponemes have a cytoplasmic and an outer membrane. Using light microscopy, treponemes are visible only by using dark field illumination. Treponema pallidum consists of three subspecies, T. p. pallidum, T. p. endemicum, and T. p. pertenue, each of which has a distinct associated disease.[4]
Subspecies
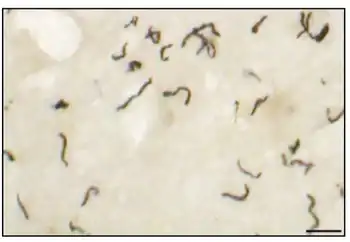
Three subspecies of T. pallidum are known:[5]
- Treponema pallidum pallidum, which causes syphilis
- T. p. endemicum, which causes bejel or endemic syphilis
- T. p. pertenue, which causes yaws
The three subspecies causing yaws, bejel, and syphilis are morphologically and serologically indistinguishable.[2] These bacteria were originally classified as members of separate species, but DNA hybridization analysis indicates they are members of the same species. Treponema carateum, the cause of pinta, remains a separate species because no isolate is available for DNA analysis.[6] Disease transmittance in subspecies T. p. endemicum and T. p. pertenue is considered non-venereal.[7] T. p. pallidum is the most invasive pathogenic subspecies while T. p. carateum is the least invasive of the subspecies. T. p. endemicum and T. p. pertenue are intermediately invasive.[8]
Microbiology
Ultrastructure
Treponema pallidum is a helically shaped bacterium with high mobility consisting of an outer membrane, peptidoglycan layer, inner membrane, protoplasmic cylinder, and periplasmic space. It is often described as Gram negative, but its outer membrane lacks lipopolysaccharide, which is found in the outer membrane of other Gram-negative bacteria.[9] It has an endoflagellum (periplasmic flagellum) consisting of four main polypeptides, a core structure, and a sheath. The flagellum is located within the periplasmic space and wraps around the protoplasmic cylinder. T. pallidum's outer membrane has the most contact with host cells and contains few transmembrane proteins, limiting antigenicity while its cytoplasmic membrane is covered in lipoproteins.[3][10] The outer membrane's treponemal ligands main function is attachment to host cells, with functional and antigenic relatedness between ligands.[11] The genus Treponema has ribbons of cytoskeletal cytoplasmic filaments that run the length of the cell just underneath the cytoplasmic membrane. They are composed of the intermediate filament-like protein CfpA (cytoplasmic filament protein A). Although the filaments may be involved in chromosome structure and segregation or cell division, their precise function is unknown.[12][10]
Outer Membrane Proteins
Treponemal outer membrane proteins are key factors for its pathogenesis, persistence and immune evasion strategies.
TP0326
TP0326 is an ortholog of BamA. BamA apparatus will insert newly synthetised and exported outer membrane proteins into the outer membrane [13]
Treponema repeat family of proteins
Treponema repeat family of proteins, Tpr for short, are proteins expressed during the infection process. Tprs are formed by a conserved N-terminal domain, an amino-terminal stretch of about 50 aminoacids, a central variable region and a conserved C-terminal domain [13].There are many different types of tpr: tprA, tprB, tprC, tprD, tprE…However, variability of tprK is the most relevant due to the immune scape characteristics it allows.
Antigen variation in TprK is regulated by gene conversion. In this way, fragments of the seven variable regions (V1–V7) present in tprK and the 53 donor sites of tprD can be combined to produce new structured sequences.[14] TprK antigen variation can help Treponema pallidum to evade a strong host immune reaction and it can also allow the reinfection of individuals. This is possible because the newly structured proteins can avoid antibody specific recognition.
In order to introduce more phenotypic diversity, Treponema pallidum may undergo phase variation. This process will mainly happen in tprF, tprI, tprG, tprJ, tprL and it consists of a reversible expansion or contraction of polymeric repeats. These size variations can help the bacterium to quickly adapt to its microenvironment, dodge immune response or even increase affinity to host.[14]
Culture
Successful long-term cultivation of T. pallidum subspecies pallidum in a tissue culture system has been reported in 2018.[15]
However, because T. pallidum cannot be grown in a pure culture, it does not satisfy Koch's Postulates.[16]
Genome
The chromosomes of the T. pallidum subspecies are small, about 1.14 Mbp. Their DNA sequences are more than 99.7% identical.[17] T. pallidum subspecies pallidum was sequenced in 1998.[18] This sequencing is significant due to T. pallidum not being capable of growing in a pure culture, meaning that this sequencing played an important role in understanding the microbe's functions. It revealed that T. pallidum relies on its host for many molecules provided by biosynthetic pathways, and that it is missing genes responsible for encoding key enzymes in oxidative phosphorylation and the tricarboxylic acid cycle. It was found that this is due to 5% of T. pallidum's genes coding for transport genes.[19] The recent sequencing of the genomes of several spirochetes permits a thorough analysis of the similarities and differences within this bacterial phylum and within the species.[20][21][22] T. p. pallidum has one of the smallest bacterial genomes at 1.14 million base pairs, and has limited metabolic capabilities, reflecting its adaptation through genome reduction to the rich environment of mammalian tissue. The shape of T. pallidum is flat and wavy.[23] In order to avoid antibodies attacking, the cell has few proteins exposed on the outer membrane sheath.[24] Its chromosome of about 1000 kilo base pairs is circular with a 52.8% G + C average.[25] Sequencing has revealed a bundle of twelve proteins and some putative hemolysins are potential virulence factors of T. pallidum.[26] 92.9% of DNA was determined to be ORF's, 55% of which had predicted biological functions.[3]
Disease
The clinical features of syphilis, yaws, and bejel occur in multiple stages that affect the skin. The skin lesions observed in the early stage last for weeks or months. The skin lesions are highly infectious, and the spirochetes in the lesions are transmitted by direct contact. The lesions regress as the immune response develops against T. pallidum. The latent stage that results lasts a lifetime in many cases. In a minority of cases, the disease exits latency and enters a tertiary phase, in which destructive lesions of skin, bone, and cartilage ensue. Unlike yaws and bejels, syphilis in its tertiary stage often affects the heart, eyes, and nervous system as well.[6]
Syphilis
Treponema pallidum pallidum is a motile spirochaete that is generally acquired by close sexual contact, entering the host via breaches in squamous or columnar epithelium. The organism can also be transmitted to a fetus by transplacental passage during the later stages of pregnancy, giving rise to congenital syphilis. The helical structure of T. p. pallidum allows it to move in a corkscrew motion through mucous membranes or enter minuscule breaks in the skin. In women the initial lesion is usually on the labia, the walls of the vagina, or the cervix; in men it is on the shaft or glans of the penis.[2] It gains access to the host's blood and lymph systems through tissue and mucous membranes. In more severe cases, it may gain access to the host by infecting the skeletal bones and central nervous system of the body.[2]
The incubation period for a T. p. pallidum infection is usually around 21 days, but can range from 10 to 90 days.[27]
Laboratory

Treponema pallidum was first microscopically identified in syphilitic chancres by Fritz Schaudinn and Erich Hoffmann at the Charité in Berlin in 1905.[28] This bacterium can be detected with special stains, such as the Dieterle stain. T. pallidum is also detected by serology, including nontreponemal VDRL, rapid plasma reagin, treponemal antibody tests (FTA-ABS), T. pallidum immobilization reaction, and syphilis TPHA test.[29]
Prevention
No vaccine for syphilis is available as of 2017. The outer membrane of T. pallidum has too few surface proteins for an antibody to be effective. Efforts to develop a safe and effective syphilis vaccine have been hindered by uncertainty about the relative importance of humoral and cellular mechanisms to protective immunity,[30] and because T. pallidum outer membrane proteins have not been unambiguously identified.[31][32] In contrast, some of the known antigens are intracellular, and antibodies are ineffective against them to clear the infection.[33][34][35]
Treatment
During the early 1940s, rabbit models in combination with the drug penicillin allowed for a long term drug treatment. These experiments establish the ground work that modern scientists use for syphilis therapy. Penicillin can inhibit T. pallidum in 6–8 hours though the cells still remain in lymph nodes and regenerate. Penicillin is not the only drug that can be used to inhibit T. pallidum; it has been found that any β-lactam antibiotics or macrolides can be used.[36] The T. pallidum strain 14 has built resistance to some macrolides, including erythromycin and azithromycin. Resistance to macrolides in T. Pallidum strain 14 is believed to derive from a single point mutation that increased the organism's livability.[37] Many of the syphilis treatment therapies only lead to bacteriostatic results, unless larger concentrations of penicillin are used for bactericidal effects.[36][37] Penicillin overall is the most recommended antibiotic by the CDC as it shows the best results with prolonged usage. It can inhibit and may even kill T. Pallidum at low to high doses with each increase in concentration being more effective.[37]
References
- ↑ Radolf, Justin D. (1996). "Treponema". Chapter 36Treponema. ncbi.nlm.nih.gov. University of Texas Medical Branch at Galveston. ISBN 9780963117212. Archived from the original on 17 August 2021. Retrieved 13 February 2019.
- 1 2 3 4 Radolf JD (1996). "Treponema". In Baron S (ed.). Medical Microbiology (4th ed.). Galveston (TX): University of Texas Medical Branch at Galveston. ISBN 978-0963117212. PMID 21413263. Archived from the original on 17 August 2021. Retrieved 15 April 2023.
- 1 2 3 Norris, Cox, Weinstock, Steven J., David L., George M. (2001). "Biology of Treponema pallidum: Correlation of Functional Activities With Genome Sequence Data" (PDF). JMMB Review. 3 (1): 37–62. PMID 11200228. Archived (PDF) from the original on 26 January 2022. Retrieved 15 April 2023.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Centurion-Lara, Arturo; Molini, Barbara J.; Godornes, Charmie; Sun, Eileen; Hevner, Karin; Voorhis, Wesley C. Van; Lukehart, Sheila A. (1 September 2006). "Molecular Differentiation of Treponema pallidum Subspecies". Journal of Clinical Microbiology. 44 (9): 3377–3380. doi:10.1128/JCM.00784-06. ISSN 0095-1137. PMC 1594706. PMID 16954278.
- ↑ Marks M, Solomon AW, Mabey DC (October 2014). "Endemic treponemal diseases". Transactions of the Royal Society of Tropical Medicine and Hygiene. 108 (10): 601–7. doi:10.1093/trstmh/tru128. PMC 4162659. PMID 25157125.
- 1 2 Giacani L, Lukehart SA (January 2014). "The endemic treponematoses". Clinical Microbiology Reviews. 27 (1): 89–115. doi:10.1128/CMR.00070-13. PMC 3910905. PMID 24396138.
- ↑ "Other Treponema pallidum infections | Immigrant and Refugee Health | CDC". www.cdc.gov. 26 February 2019. Archived from the original on 15 April 2023. Retrieved 12 November 2019.
- ↑ Radolf, Justin D. (1996), Baron, Samuel (ed.), "Treponema", Medical Microbiology (4th ed.), University of Texas Medical Branch at Galveston, ISBN 9780963117212, PMID 21413263, archived from the original on 17 August 2022, retrieved 12 November 2019
- ↑ Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS (October 2017). "Syphilis". Nature Reviews. Disease Primers. 3: 17073. doi:10.1038/nrdp.2017.73. PMC 5809176. PMID 29022569.
- 1 2 Liu J, Howell JK, Bradley SD, Zheng Y, Zhou ZH, Norris SJ (November 2010). "Cellular architecture of Treponema pallidum: novel flagellum, periplasmic cone, and cell envelope as revealed by cryo electron tomography". Journal of Molecular Biology. 403 (4): 546–61. doi:10.1016/j.jmb.2010.09.020. PMC 2957517. PMID 20850455.
- ↑ Alderete, John F.; Baseman, Joel B. (1 December 1980). "Surface Characterization of Virulent Treponema pallidum". Infection and Immunity. 30 (3): 814–823. doi:10.1128/iai.30.3.814-823.1980. ISSN 0019-9567. PMC 551388. PMID 7014451.
- ↑ Izard J (2006). "Cytoskeletal cytoplasmic filament ribbon of Treponema: a member of an intermediate-like filament protein family". Journal of Molecular Microbiology and Biotechnology. 11 (3–5): 159–66. doi:10.1159/000094052. PMID 16983193. S2CID 40913042.
- 1 2 Hawley, Kelly L.; Montezuma-Rusca, Jairo M.; Delgado, Kristina N.; Singh, Navreeta; Uversky, Vladimir N.; Caimano, Melissa J.; Radolf, Justin D.; Luthra, Amit (8 July 2021). Galperin, Michael Y. (ed.). "Structural Modeling of the Treponema pallidum Outer Membrane Protein Repertoire: a Road Map for Deconvolution of Syphilis Pathogenesis and Development of a Syphilis Vaccine". Journal of Bacteriology. 203 (15): e0008221. doi:10.1128/JB.00082-21. ISSN 0021-9193. PMC 8407342. PMID 33972353.
- 1 2 Tang, Yun; Zhou, Yingjie; He, Bisha; Cao, Ting; Zhou, Xiangping; Ning, Lichang; Chen, En; Li, Yumeng; Xie, Xiaoping; Peng, Binfeng; Hu, Yibao; Liu, Shuangquan (19 October 2022). "Investigation of the immune escape mechanism of Treponema pallidum". Infection. doi:10.1007/s15010-022-01939-z. ISSN 1439-0973. PMID 36260281. S2CID 252994863. Archived from the original on 26 April 2023. Retrieved 15 April 2023.
- ↑ Edmondson DG, Hu B, Norris SJ (June 2018). "Long-Term in Vitro Culture of the Syphilis Spirochete Treponema pallidum subsp. pallidum". mBio. 9 (3). doi:10.1128/mBio.01153-18. PMC 6020297. PMID 29946052.
- ↑ Prescott, Joseph; Feldmann, Heinz; Safronetz, David (January 2017). "Amending Koch's postulates for viral disease: When "growth in pure culture" leads to a loss of virulence". Antiviral Research. 137: 1–5. doi:10.1016/j.antiviral.2016.11.002. ISSN 0166-3542. PMC 5182102. PMID 27832942. Archived from the original on 26 April 2023. Retrieved 15 April 2023.
- ↑ Šmajs D, Strouhal M, Knauf S (July 2018). "Genetics of human and animal uncultivable treponemal pathogens". Infection, Genetics and Evolution. 61: 92–107. doi:10.1016/j.meegid.2018.03.015. PMID 29578082. S2CID 4826749.
- ↑ Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, et al. (July 1998). "Complete genome sequence of Treponema pallidum, the syphilis spirochete". Science. 281 (5375): 375–88. Bibcode:1998Sci...281..375F. doi:10.1126/science.281.5375.375. PMID 9665876. S2CID 8641048. Archived from the original on 19 April 2023. Retrieved 15 April 2023.
- ↑ Willey, Joanne M. (2020). Prescott's Microbiology, Eleventh Edition. New York: McGraw-Hill Education. p. 436. ISBN 978-1-260-21188-7.
- ↑ Zobaníková M, Mikolka P, Cejková D, Pospíšilová P, Chen L, Strouhal M, Qin X, Weinstock GM, Smajs D (October 2012). "Complete genome sequence of Treponema pallidum strain DAL-1". Standards in Genomic Sciences. 7 (1): 12–21. doi:10.4056/sigs.2615838. PMC 3570794. PMID 23449808.
- ↑ Tong ML, Zhao Q, Liu LL, Zhu XZ, Gao K, Zhang HL, Lin LR, Niu JJ, Ji ZL, Yang TC (2017). "Whole genome sequence of the Treponema pallidum subsp. pallidum strain Amoy: An Asian isolate highly similar to SS14". PLOS ONE. 12 (8): e0182768. Bibcode:2017PLoSO..1282768T. doi:10.1371/journal.pone.0182768. PMC 5546693. PMID 28787460.
- ↑ Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, Dodson RJ, et al. (April 2004). "Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes". Proceedings of the National Academy of Sciences of the United States of America. 101 (15): 5646–51. Bibcode:2004PNAS..101.5646S. doi:10.1073/pnas.0307639101. PMC 397461. PMID 15064399.
- ↑ Clark DP, Dunlap PV, Madigan JT, Martinko JM (2009). Brock Biology of Microorganisms. San Francisco: Pearson. p. 79.
- ↑ Willey, Joanne M. (2020). Prescott's Microbiology (11th ed.). New York: McGraw-Hill Education. p. 499. ISBN 978-1-260-21188-7.
- ↑ Fraser, Claire M.; Norris, Steven J.; Weinstock, George M.; White, Owen; Sutton, Granger G.; Dodson, Robert; Gwinn, Michelle; Hickey, Erin K.; Clayton, Rebecca; Ketchum, Karen A.; Sodergren, Erica (17 July 1998). "Complete Genome Sequence of Treponema pallidum, the Syphilis Spirochete". Science. 281 (5375): 375–388. Bibcode:1998Sci...281..375F. doi:10.1126/science.281.5375.375. ISSN 0036-8075. PMID 9665876. S2CID 8641048. Archived from the original on 19 April 2023. Retrieved 15 April 2023.
- ↑ Weinstock, George M.; Hardham, John M.; McLeod, Michael P.; Sodergren, Erica J.; Norris, Steven J. (1 October 1998). "The genome of Treponema pallidum: new light on the agent of syphilis". FEMS Microbiology Reviews. 22 (4): 323–332. doi:10.1111/j.1574-6976.1998.tb00373.x. ISSN 0168-6445. PMID 9862125.
- ↑ "STD Facts – Syphilis (Detailed)". Centers for Disease Control (CDC). Archived from the original on 30 July 2018. Retrieved 19 April 2017.
- ↑ Schaudinn FR, Hoffmann E (1905). "Vorläufiger Bericht über das Vorkommen von Spirochaeten in syphilitischen Krankheitsprodukten und bei Papillomen" [Preliminary report on the occurrence of Spirochaetes in syphilitic chancres and papillomas]. Arbeiten aus dem Kaiserlichen Gesundheitsamte. 22: 527–534.
- ↑ Fisher B, Harvey RP, Champe PC (2007). Lippincott's Illustrated Reviews: Microbiology (Lippincott's Illustrated Reviews Series). Hagerstown, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-8215-9.
- ↑ Bishop NH, Miller JN (July 1976). "Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization". Journal of Immunology. 117 (1): 191–6. doi:10.4049/jimmunol.117.1.191. PMID 778261. S2CID 255333392. Archived from the original on 20 April 2022. Retrieved 15 April 2023.
{{cite journal}}: Check|s2cid=value (help) - ↑ Tomson FL, Conley PG, Norgard MV, Hagman KE (September 2007). "Assessment of cell-surface exposure and vaccinogenic potentials of Treponema pallidum candidate outer membrane proteins". Microbes and Infection. 9 (11): 1267–75. doi:10.1016/j.micinf.2007.05.018. PMC 2112743. PMID 17890130.
- ↑ Cameron CE, Lukehart SA (March 2014). "Current status of syphilis vaccine development: need, challenges, prospects". Vaccine. 32 (14): 1602–9. doi:10.1016/j.vaccine.2013.09.053. PMC 3951677. PMID 24135571.
- ↑ Penn CW, Bailey MJ, Cockayne A (April 1985). "The axial filament antigen of Treponema pallidum". Immunology. 54 (4): 635–41. PMC 1453562. PMID 3884491.
- ↑ Norris SJ (September 1993). "Polypeptides of Treponema pallidum: progress toward understanding their structural, functional, and immunologic roles. Treponema Pallidum Polypeptide Research Group". Microbiological Reviews. 57 (3): 750–79. doi:10.1128/MMBR.57.3.750-779.1993. PMC 372934. PMID 8246847.
- ↑ Izard J, Renken C, Hsieh CE, Desrosiers DC, Dunham-Ems S, La Vake C, Gebhardt LL, Limberger RJ, Cox DL, Marko M, Radolf JD (December 2009). "Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum, the syphilis spirochete". Journal of Bacteriology. 191 (24): 7566–80. doi:10.1128/JB.01031-09. PMC 2786590. PMID 19820083.
- 1 2 Fantry, Lori E; Tramont, Edmund C. "Treponema Pallidum (Syphilis) - Infectious Disease and Antimicrobial Agents". www.antimicrobe.org. Archived from the original on 12 March 2015. Retrieved 12 November 2019.
- 1 2 3 Stamm, Lola V. (1 February 2010). "Global Challenge of Antibiotic-Resistant Treponema pallidum". Antimicrobial Agents and Chemotherapy. 54 (2): 583–589. doi:10.1128/AAC.01095-09. ISSN 0066-4804. PMC 2812177. PMID 19805553.
Further reading
- Althouse BM, Hébert-Dufresne L (October 2014). "Epidemic cycles driven by host behaviour". Journal of the Royal Society, Interface. 11 (99): 20140575. doi:10.1098/rsif.2014.0575. PMC 4235258. PMID 25100316.
External links
- "Syphilis- CDC Fact Sheet Archived 11 February 2013 at the Wayback Machine." Centers for Disease Control and Prevention. May. 2004. Centers for Disease Control and Prevention. 7 February 2006