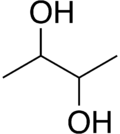
2,3-Butanediol
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butane-2,3-diol | |
| Other names
2,3-Butylene glycol Dimethylene glycol 2,3-Dihydroxybutane Butan-2,3-diol Diethanol & Bis-ethanol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.431 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C4H10O2 |
| Molar mass | 90.122 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | odorless |
| Density | 0.987 g/mL |
| Melting point | 19 °C (66 °F; 292 K) |
| Boiling point | 177 °C (351 °F; 450 K) |
Solubility in water |
Miscible |
| Solubility in other solvents | Soluble in alcohol, ketones, ether |
| log P | -0.92 |
| Vapor pressure | 0.23 hPa (20 °C) |
| Acidity (pKa) | 14.9 |
Refractive index (nD) |
1.4366 |
| Thermochemistry | |
Heat capacity (C) |
213.0 J/K mol |
Std enthalpy of formation (ΔfH⦵298) |
-544.8 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 85 °C (185 °F; 358 K) |
Autoignition temperature |
402 °C (756 °F; 675 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
5462 mg/kg (rat, oral) |
| Related compounds | |
Related butanediols |
1,4-Butanediol 1,3-Butanediol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,3-Butanediol is the organic compound with the formula (CH3CHOH)2. It is classified as a vic-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids. Applications include precursors to various plastics and pesticides.
Isomerism
Of the three stereoisomers, two are enantiomers (levo- and dextro-2,3-butanediol) and one is a meso compound.[1][2] The enantiomeric pair have (2R, 3R) and (2S, 3S) configurations at carbons 2 and 3, while the meso compound has configuration (2R, 3S) or, equivalently, (2S, 3R).
Industrial production and uses
2,3-Butanediol is prepared by hydrolysis of 2,3-epoxybutane:[3]
- (CH3CH)2O + H2O → CH3(CHOH)2CH3
The isomer distribution depends on the stereochemistry of the epoxide.
The meso isomer is used to combine with naphthalene-1,5-diisocyanate. The resulting polyurethane is called "Vulkollan".[3]
Biological production
The (2R,3R)-stereoisomer of 2,3-butanediol is produced by a variety of microorganisms in a process known as butanediol fermentation.[4] It is found naturally in cocoa butter, in the roots of Ruta graveolens, sweet corn, and in rotten mussels. It is used in the resolution of carbonyl compounds in gas chromatography.[5]
During World War II research was done towards producing 2,3-butanediol by fermentation in order to produce 1,3-butadiene, the monomer of the polybutadiene used in a leading type of synthetic rubber.[6] It can be derived from the fermentation of sugarcane molasses.[7]
Fermentative production of 2,3-butanediol from carbohydrates involves a network of biochemical reactions that can be manipulated to maximize production.[8]
2,3-butanediol has been proposed as a rocket fuel that could be created on Mars by means of cyanobacteria and E. coli, shipped from Earth, working on resources available at the surface of Mars.[9]
Reactions
2,3-Butanediol undergo dehydration to form butanone (methyl ethyl ketone):[10]
- (CH3CHOH)2 → CH3C(O)CH2CH3 + H2O
It can also undergo deoxydehydration to form butene:[11]
- (CH3CHOH)2 + 2 H2 → C4H8 + 2 H2O
References
- ↑ Boutron P (1992). "Cryoprotection of red blood cells by a 2,3-butanediol containing mainly the levo and dextro isomers". Cryobiology. 29 (3): 347–358. doi:10.1016/0011-2240(92)90036-2. PMID 1499320.
- ↑ Wang Y, Tao F, Xu P (2014). "Glycerol dehydrogenase plays a dual role in glycerol metabolism and 2,3-butanediol formation in Klebsiella pneumoniae". Journal of Biological Chemistry. 289 (9): 6080–6090. doi:10.1074/jbc.M113.525535. PMC 3937674. PMID 24429283.
- 1 2 Heinz Gräfje, Wolfgang Körnig, Hans-Martin Weitz, Wolfgang Reiß, Guido Steffan, Herbert Diehl, Horst Bosche, Kurt Schneider and Heinz Kieczka "Butanediols, Butenediol, and Butynediol" in Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_455
- ↑ C. De Mas; N. B. Jansen; G. T. Tsao (1988). "Production of optically active 2,3-butanediol by Bacillus polymyxa". Biotechnol. Bioeng. 31 (4): 366–377. doi:10.1002/bit.260310413. PMID 18584617. S2CID 36530193.
- ↑ "3,5-dinitrobenzoic acid". Combined Chemical Dictionary. Chapman and Hall/CRC Press. 2007.
- ↑ "Fermentation Derived 2,3-Butanediol", by Marcio Voloch et al. in Comprehensive Biotechnology, Pergamon Press Ltd., England Vol 2, Section 3, p. 933 (1986).
- ↑ Dai, Jian-Ying; Zhao, Pan; Cheng, Xiao-Long; Xiu, Zhi-Long (2015). "Enhanced Production of 2,3-Butanediol from Sugarcane Molasses". Applied Biochemistry and Biotechnology. 175 (6): 3014–3024. doi:10.1007/s12010-015-1481-x. ISSN 0273-2289. PMID 25586489. S2CID 11287904.
- ↑ Jansen, Norman B.; Flickinger, Michael C.; Tsao, George T. (1984). "Application of bioenergetics to modelling the microbial conversion of D-xylose to 2,3-butanediol". Biotechnol Bioeng. 26 (6): 573–582. doi:10.1002/bit.260260603. PMID 18553372. S2CID 22878894.
- ↑ "Rocket fuel made on Mars could propel astronauts back to Earth", Design Products & Applications, accessed 6 December 2021.
- ↑ Nikitina, Maria A.; Ivanova, Irina I. (2016-02-23). "Conversion of 2,3-Butanediol over Phosphate Catalysts". ChemCatChem. 8 (7): 1346–1353. doi:10.1002/cctc.201501399. ISSN 1867-3880. S2CID 102135312.
- ↑ Kwok, Kelvin Mingyao; Choong, Catherine Kai Shin; Ong, Daniel Sze Wei; Ng, Joy Chun Qi; Gwie, Chuandayani Gunawan; Chen, Luwei; Borgna, Armando (2017-06-07). "Hydrogen-Free Gas-Phase Deoxydehydration of 2,3-Butanediol to Butene on Silica-Supported Vanadium Catalysts". ChemCatChem. 9 (13): 2443–2447. doi:10.1002/cctc.201700301. ISSN 1867-3880. S2CID 99415384.
