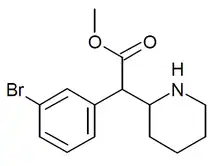
3-Bromomethylphenidate
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C14H18BrNO2 |
| Molar mass | 312.207 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
3-Bromomethylphenidate (3-Br-MPH) is a compound from the phenidate family, which has reportedly been sold as a designer drug. It showed the most potent binding to the dopamine transporter of a series of ring-substituted methylphenidate derivatives, and produced stimulant effects in animal studies.[1][2][3]
See also
References
- ↑ Pan D, Gatley SJ, Dewey SL, Chen R, Alexoff DA, Ding YS, Fowler JS (October 1994). "Binding of bromine-substituted analogs of methylphenidate to monoamine transporters". European Journal of Pharmacology. 264 (2): 177–82. doi:10.1016/0014-2999(94)00460-9. PMID 7851480.
- ↑ Deutsch HM, Shi Q, Gruszecka-Kowalik E, Schweri MM (March 1996). "Synthesis and pharmacology of potential cocaine antagonists. 2. Structure-activity relationship studies of aromatic ring-substituted methylphenidate analogs". Journal of Medicinal Chemistry. 39 (6): 1201–9. doi:10.1021/jm950697c. PMID 8632426.
- ↑ Misra M, Shi Q, Ye X, Gruszecka-Kowalik E, Bu W, Liu Z, et al. (October 2010). "Quantitative structure-activity relationship studies of threo-methylphenidate analogs". Bioorganic & Medicinal Chemistry. 18 (20): 7221–38. doi:10.1016/j.bmc.2010.08.034. PMID 20846865.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.