AVP gene
Arginine Vasopressin (AVP) Gene is a gene whose product is proteolytically cleaved to produce vasopressin (also known as antidiuretic hormone, ADH), neurophysin II, and a glycoprotein called copeptin. AVP and other AVP-like peptides are found in mammals, as well as mollusks, arthropods, nematodes, and other invertebrate species.[5] In humans, AVP is present on chromosome 20 and plays a role in homeostatic regulation. The products of AVP have many functions that include vasoconstriction, regulating the balance of water in the body, and regulating responses to stress.[6] Expression of AVP is regulated by the Transcription Translation Feedback Loop (TTFL), which is an important part of the circadian system that controls the expression of clock genes. AVP has important implications in the medical field as its products have significant roles throughout body.
Discovery
Vasopressin
The discovery of the AVP gene first required the discovery of one of its key products: vasopressin. In 1895, G. Oliver and E.A. Schäfer found that a substance released by the pituitary gland could elevate blood pressure. The researchers noted that intravenous injection of extracts from the pituitary gland, thyroid gland, and spleen all influence blood pressure, however the effect from the pituitary had the most significant impact.[7] Almost thirty years later, Kamm and colleagues separated the components within the pituitary gland. Using a unique, five-step separation technique, Kamm revealed one substance associated with uterine contractions – oxytocin – and another substance associated with blood pressure – vasopressin.[8]
Once the discovery and separation of vasopressin occurred, subsequent research on the product's structure, function, and mode of generation could take place. In 1951, Turner and colleagues uncovered the amino acid sequence behind the hormone. The amino acid structure was composed of eight amino acids, including phenylalanine, tyrosine, proline, glutamic acid, aspartic acid, glycine, arginine, and cystine. The structure was also found to include ammonia.[9] Following this discovery, Vincent du Vigneaud was able to synthesize a synthetic form of vasopressin in a laboratory setting. Du Vigneaud specifically noted that his final product had the same activity and composition ratios as that of naturally occurring vasopressin.[10]
AVP gene
The work on vasopressin ultimately allowed researchers to work backwards and identify the gene responsible for this product's generation. This final stage of research began when Gainer and colleagues found a precursor protein to vasopressin in 1977.[11] The structure of the protein was subsequently discovered by Land in 1982. By sequencing complementary DNA strands that encoded for the hormone's mRNA, Land outlined the amino acid sequence of the precursor protein.[12] Finally, one year later, Schmale, Heinsohn, and Richter isolated the AVP precursor gene in rats from their genomic library. The researchers used restriction mapping and nucleotide sequence analysis to uncover the gene's three distinct exons and the products (vasopressin, neurophysin, and glycoprotein) that each was responsible for.[13]
Structure
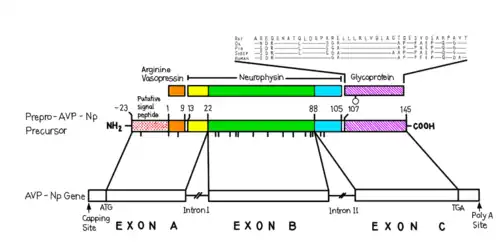
The 1.85 kilobase-long AVP gene, located on chromosome 20 (20p13) contains three functional domains, including AVP, neurophysin II (NP) and a C-terminal glycopeptide called copeptin. Using restriction mapping and sequencing, the gene was found to have these domains which spanned over three exons with two intronic sequences. Exon A encodes a putative signal peptide, the arginine vasopressin hormone, and the N terminus of the NP carrier protein. Exon B, which is separated from exon A with a 1 kilobase-long intron, encodes the conserved middle portion of NP. A 227 kilobase intron separates exon B from exon C, which encodes the final domain, including the C terminus of NP and the glycoprotein. The structure of this gene has been found to be generally conserved across species, including chimpanzees, Rhesus monkeys, dogs, cows, mice, rats, chicken, zebrafish, and frogs.[13]
Promoter region
The AVP gene promoter region consists of an E-box element located 150 residues upstreams of the transcription start site, which binds mammalian clock proteins CLOCK and BMAL1 involved in generating circadian rhythms in the suprachiasmatic nucleus (SCN).[14] BMAL1 and CLOCK gene knockouts in the SCN (Bmal-/- and clk-/-) eliminate rhythmicity in AVP mRNA expression, confirming that binding of the protein heterodimers to the E-box element is necessary for the intrinsic circadian pattern of the AVP gene.[15] In addition to the E-box element, the promoter region of the AVP gene also contains a cAMP response element (CRE) site that is involved in gene expression regulation. Daily rhythms in the phosphorylation of the CRE binding protein (CREB) supports that these elements also contribute to circadian rhythmicity of the gene expression. CRE/CREB-mediated regulation of the AVP gene is activated through the cAMP activation of Ras signaling pathways, culminating in the MAP kinase phosphorylation of the CREB transcription factor.[14]
Transcription of the AVP gene to produce AVP mRNA has daily rhythms, with mRNA levels peaking during the subjective day and reaching their lowest point in the subject night. This rhythm is regulated by the binding of circadian proteins to the E-box, along with transcriptional regulation of other elements, including the CRE in the promoter region.[14]
Function
AVP is primarily known for its role as a mammalian circadian output.[15][16] The most common product of AVP is vasopressin, which is a neurohypophysial hormone that is important in homeostatic mechanisms and processes, like cortical EEG rhythms. Its other products are neurophysin II and copeptin. AVP is produced in a specific type of neuron called magnocellular neurons (MCNs), which are located in the hypothalamus.[16] In mammals, the AVP gene is also transcribed in the suprachiasmatic nucleus where its expression is under the control of the circadian TTFL.[17] It is well established that the finished vasopressin product is transported from the cell body to the terminals in the posterior pituitary gland where it is released into the bloodstream as a result of environmental stressors like dehydration.[18]
Gene Regulation
AVP gene regulation is controlled by the TTFL. In this system, per2 protein is transcribed and phosphorylated by the CK1E enzyme. Accumulation of this protein inhibits the transcription factors Clock and BMAL1, so that no additional per product is created.[19] At the same time, per2 inhibits the transcription factors driving the AVP gene so that its expression and products are reduced.[18] It can be noted that AVP expression is regulated by the TTFL in the SCN but not in the paraventricular nucleus of the hypothalamus (PVH) and supraoptic nucleus (SON).[20]
Receptor Pathways
The transcription of the AVP gene commonly results in the binding of vasopressin to one of three vasopressin receptors: AVPR1A, AVPR1B, and AVPR2. When vasopressin binds to AVPR1A, a G-protein coupled receptor (GPCR), phospholipase C (PLC) becomes activated.[21][22] This pathway typically involves regulating vasoconstriction. When vasopressin binds to AVPR1B, a GPCR, the phosphatidylinositol-calcium second messenger system is stimulated. AVPR1A and AVPR1B are GPCRs that stimulate PLC to promote the production of DAG which activates PKC and IP3 and stimulates calcium ion release from the ER. This signaling pathway is important in regulating homeostasis and the amount of water, glucose, and salts within the blood via ACTH release and storage.[23] When vasopressin binds to AVPR2, another GPCR, adenylyl cyclase is stimulated. This second messenger pathway involves the regulation of ADH, or vasopressin, in the kidneys, which has an important diuretic purpose of retaining water and manipulating the concentrations of soluble toxic wastes and urea in urine.[24]
AVP gene in rats
Within rats, the AVP gene is important for the regulation of various processes associated with the excretory system and smooth muscle cells. The AVP gene and arginine vasopressin are commonly colocalized with oxytocin because synaptic transmission of oxytocin regulates the expression of AVP mRNA.[25]
In one study conducted by Greenwood and colleagues, researchers found that AVP gene expression in rats is regulated by the cAMP responsive element-binding protein-3 like-1 (CREB3L1). CREB3L1 is activated when it is cleaved and when the AVP gene is translocated from the Golgi to the nucleus.[26] Additionally, CREB3L1 mRNA levels correspond with increased amounts of transcription of the AVP gene in the hypothalamus following a deficiency of sodium and as a consequence of diurnal rhythm in the SCN.[26] Both full-length and constitutively active forms of CREB3L1 (CREB3L1CA) induce the expression of rat AVP promoter-luciferase reporter constructs whereas a dominant-negative mutant reduces expression. From this study, the researchers concluded that CREB3L1 is a regulator of AVP gene transcription in the hypothalamus and specifically within the PVH and SON.
Arginine vasopressin stimulates the process of phosphorylation of aquaporin 2 (AQP2) of renal tissue which contributes to the overall increased permeability of water in the collecting duct cells of the tissue. The phosphorylation of AQP2 leads to activation of the protein kinase A signaling pathway which amplifies the permeability of water by stimulating the rat equivalent of the urea transporter 1 protein.[27]
Medical applications
Main Article: Vasopressin (medication)
The functions of vasopressin make it useful for a variety of important medical applications. Since it plays a role in the regulation of many physiological functions, like regulation of water and sodium excretion, blood volume, vasoconstriction, and response to stress, vasopressin can be helpful in the treatment of conditions related to these functions.[28] These applications include treatment of nocturnal enuresis, diabetes insipidus, and hemophilia A.[29] Additionally, it is used to treat some forms of shock including septic shock and vasodilatory shock. It is also used during surgery to decrease blood loss.[30]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000101200 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000037727 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Albers HE (January 2015). "Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network". Frontiers in Neuroendocrinology. 36: 49–71. doi:10.1016/j.yfrne.2014.07.001. PMC 4317378. PMID 25102443.
- ↑ Ryckmans T (September 2010). "Modulation of the vasopressin system for the treatment of CNS diseases". Current Opinion in Drug Discovery & Development. 13 (5): 538–47. PMID 20812145.
- ↑ Oliver G, Schäfer EA (July 1895). "On the Physiological Action of Extracts of Pituitary Body and certain other Glandular Organs: Preliminary Communication". The Journal of Physiology. 18 (3): 277–9. doi:10.1113/jphysiol.1895.sp000565. PMC 1514634. PMID 16992253.
- ↑ Kamm O, Aldrich TB, Grote IW, Rowe LW, Bugbee EP (1928-02-01). "The Active Principles of the Posterior Lobe of the Pituitary Gland.1 I. The Demonstration of the Presence of Two Active Principles. Ii. The Separation of the Two Principles and Their Concentration in the Form of Potent Solid Preparations". Journal of the American Chemical Society. 50 (2): 573–601. doi:10.1021/ja01389a050. ISSN 0002-7863.
- ↑ Turner RA, Pierce JG, du Vigneaud V (July 1951). "The purification and the amino acid content of vasopressin preparations". The Journal of Biological Chemistry. 191 (1): 21–8. doi:10.1016/S0021-9258(18)50947-9. PMID 14850440.
- ↑ du Vigneaud V, Gish DT, Katsoyannis PG (1954-09-01). "A Synthetic Preparation Possessing Biological Properties Associated with Argininevasopressin". Journal of the American Chemical Society. 76 (18): 4751–4752. doi:10.1021/ja01647a089. ISSN 0002-7863.
- ↑ Gainer H, Sarne Y, Brownstein MJ (March 1977). "Neurophysin biosynthesis: conversion of a putative precursor during axonal transport". Science. 195 (4284): 1354–6. Bibcode:1977Sci...195.1354G. doi:10.1126/science.65791. PMID 65791. S2CID 22281152.
- ↑ Land H, Schütz G, Schmale H, Richter D (January 1982). "Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor". Nature. 295 (5847): 299–303. Bibcode:1982Natur.295..299L. doi:10.1038/295299a0. PMID 6276766. S2CID 4340962.
- 1 2 Schmale H, Heinsohn S, Richter D (1983). "Structural organization of the rat gene for the arginine vasopressin-neurophysin precursor". The EMBO Journal. 2 (5): 763–7. doi:10.1002/j.1460-2075.1983.tb01497.x. PMC 555182. PMID 6315416.
- 1 2 3 Arima H, House SB, Gainer H, Aguilera G (November 2002). "Neuronal activity is required for the circadian rhythm of vasopressin gene transcription in the suprachiasmatic nucleus in vitro". Endocrinology. 143 (11): 4165–71. doi:10.1210/en.2002-220393. PMID 12399408.
- 1 2 Mieda M (2019-02-25). "The Network Mechanism of the Central Circadian Pacemaker of the SCN: Do AVP Neurons Play a More Critical Role Than Expected?". Frontiers in Neuroscience. 13: 139. doi:10.3389/fnins.2019.00139. PMC 6397828. PMID 30858797.
- 1 2 Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R (1999-01-01). "Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus". Progress in Brain Research. 119: 351–64. doi:10.1016/S0079-6123(08)61580-0. ISBN 9780444500809. PMID 10074799.
- ↑ Antunes-Rodrigues J, de Castro M, Elias LL, Valença MM, McCann SM (January 2004). "Neuroendocrine control of body fluid metabolism". Physiological Reviews. 84 (1): 169–208. doi:10.1152/physrev.00017.2003. PMID 14715914.
- 1 2 Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM (January 1999). "A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock". Cell. 96 (1): 57–68. doi:10.1016/s0092-8674(00)80959-9. PMID 9989497. S2CID 6916996.
- ↑ Dunlap JC (January 1999). "Molecular bases for circadian clocks". Cell. 96 (2): 271–90. doi:10.1016/s0092-8674(00)80566-8. PMID 9988221. S2CID 14991100.
- ↑ "AVP arginine vasopressin [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2021-05-02.
- ↑ Bourque CW (July 2008). "Central mechanisms of osmosensation and systemic osmoregulation". Nature Reviews. Neuroscience. 9 (7): 519–31. doi:10.1038/nrn2400. PMID 18509340. S2CID 205504313.
- ↑ Caldwell HK, Lee HJ, Macbeth AH, Young WS (January 2008). "Vasopressin: behavioral roles of an "original" neuropeptide". Progress in Neurobiology. 84 (1): 1–24. doi:10.1016/j.pneurobio.2007.10.007. PMC 2292122. PMID 18053631.
- ↑ Thibonnier M, Auzan C, Madhun Z, Wilkins P, Berti-Mattera L, Clauser E (February 1994). "Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor". The Journal of Biological Chemistry. 269 (5): 3304–10. doi:10.1016/S0021-9258(17)41863-1. PMID 8106369.
- ↑ Holmes CL, Landry DW, Granton JT (December 2003). "Science review: Vasopressin and the cardiovascular system part 1--receptor physiology". Critical Care. 7 (6): 427–34. doi:10.1186/cc2337. PMC 374366. PMID 14624682.
- ↑ Baldino F, O'Kane TM, Fitzpatrick-McElligott S, Wolfson B (August 1988). "Coordinate hormonal and synaptic regulation of vasopressin messenger RNA". Science. 241 (4868): 978–81. Bibcode:1988Sci...241..978B. doi:10.1126/science.3406747. PMID 3406747.
- 1 2 Greenwood M, Bordieri L, Greenwood MP, Rosso Melo M, Colombari DS, Colombari E, et al. (March 2014). "Transcription factor CREB3L1 regulates vasopressin gene expression in the rat hypothalamus". The Journal of Neuroscience. 34 (11): 3810–20. doi:10.1523/JNEUROSCI.4343-13.2014. PMC 3951688. PMID 24623760.
- ↑ Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, Nielsen S, Nairn AC (February 1999). "Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue". The American Journal of Physiology. 276 (2): F254-9. doi:10.1152/ajprenal.1999.276.2.F254. PMID 9950956.
- ↑ Gassanov N, Semmo N, Semmo M, Nia AM, Fuhr U, Er F (April 2011). "Arginine vasopressin (AVP) and treatment with arginine vasopressin receptor antagonists (vaptans) in congestive heart failure, liver cirrhosis and syndrome of inappropriate antidiuretic hormone secretion (SIADH)" (PDF). European Journal of Clinical Pharmacology. 67 (4): 333–346. doi:10.1007/s00228-011-1006-7. PMID 21327910. S2CID 1477146.
- ↑ Agrawal A, Singh VK, Varma A, Sharma R (April 2012). "Therapeutic applications of vasopressin in pediatric patients". Indian Pediatrics. 49 (4): 297–305. doi:10.1007/s13312-012-0046-0. PMID 22565074. S2CID 9836092.
- ↑ Frishman G (February 2009). "Vasopressin: if some is good, is more better?". Obstetrics and Gynecology. 113 (2 Pt 2): 476–477. doi:10.1097/AOG.0b013e31819698bb. PMID 19155925.