Activin and inhibin
| inhibin, alpha | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | INHA | ||||||
| NCBI gene | 3623 | ||||||
| HGNC | 6065 | ||||||
| OMIM | 147380 | ||||||
| RefSeq | NM_002191 | ||||||
| UniProt | P05111 | ||||||
| Other data | |||||||
| Locus | Chr. 2 q33-qter | ||||||
| |||||||
| inhibin, beta A | |||||||
|---|---|---|---|---|---|---|---|
 The Activin dimer, from 2ARV.pdb | |||||||
| Identifiers | |||||||
| Symbol | INHBA | ||||||
| Alt. symbols | activin A | ||||||
| NCBI gene | 3624 | ||||||
| HGNC | 6066 | ||||||
| OMIM | 147290 | ||||||
| RefSeq | NM_002192 | ||||||
| UniProt | P08476 | ||||||
| Other data | |||||||
| Locus | Chr. 7 p15-p13 | ||||||
| |||||||
| inhibin, beta B | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | INHBB | ||||||
| Alt. symbols | activin B | ||||||
| NCBI gene | 3625 | ||||||
| HGNC | 6067 | ||||||
| OMIM | 147390 | ||||||
| RefSeq | NM_002193 | ||||||
| UniProt | P09529 | ||||||
| Other data | |||||||
| Locus | Chr. 2 cen-q13 | ||||||
| |||||||
| inhibin, beta C | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | INHBC | ||||||
| Alt. symbols | activin C | ||||||
| NCBI gene | 3626 | ||||||
| HGNC | 6068 | ||||||
| OMIM | 601233 | ||||||
| RefSeq | NM_005538 | ||||||
| UniProt | P55103 | ||||||
| Other data | |||||||
| Locus | Chr. 12 q13 | ||||||
| |||||||
| inhibin, beta E | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | INHBE | ||||||
| Alt. symbols | activin E | ||||||
| NCBI gene | 83729 | ||||||
| HGNC | 24029 | ||||||
| OMIM | 612031 | ||||||
| RefSeq | NM_031479 | ||||||
| UniProt | P58166 | ||||||
| Other data | |||||||
| Locus | Chr. 12 q13.2 | ||||||
| |||||||
Activin and inhibin are two closely related protein complexes that have almost directly opposite biological effects. Identified in 1986,[1][2] activin enhances FSH biosynthesis and secretion, and participates in the regulation of the menstrual cycle. Many other functions have been found to be exerted by activin, including roles in cell proliferation, differentiation, apoptosis,[3] metabolism, homeostasis, immune response, wound repair,[4] and endocrine function. Conversely, inhibin downregulates FSH synthesis and inhibits FSH secretion.[5] The existence of inhibin was hypothesized as early as 1916; however, it was not demonstrated to exist until Neena Schwartz and Cornelia Channing's work in the mid 1970s, after which both proteins were molecularly characterized ten years later.[6]
Activin is a dimer composed of two identical or very similar beta subunits. Inhibin is also a dimer wherein the first component is a beta subunit similar or identical to the beta subunit in activin. However, in contrast to activin, the second component of the inhibin dimer is a more distantly-related alpha subunit.[7][8] Activin, inhibin and a number of other structurally related proteins such as anti-Müllerian hormone, bone morphogenetic protein, and growth differentiation factor belong to the TGF-β protein superfamily.[9]
Structure
The activin and inhibin protein complexes are both dimeric in structure, and, in each complex, the two monomers are linked to one another by a single disulfide bond.[10] In addition, both complexes are derived from the same family of related genes and proteins but differ in their subunit composition.[7] Below is a list of the most common inhibin and activin complexes and their subunit composition:
|
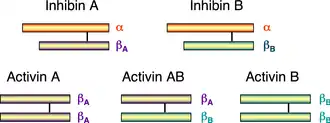 Schematic diagram of the 1D structures of inhibin and activin. The black line between the monomers represents a disulfide bond.
| |||||||||||||||||||||||||||
The alpha and beta subunits share approximately 25% sequence similarity, whereas the similarity between beta subunits is approximately 65%.[9]
In mammals, four beta subunits have been described, called activin βA, activin βB, activin βC and activin βE. Activin βA and βB are identical to the two beta subunits of inhibin. A fifth subunit, activin βD, has been described in Xenopus laevis. Two activin βA subunits give rise to activin A, one βA, and one βB subunit gives rise to activin AB, and so on. Various, but not all theoretically possible, heterodimers have been described.[11][12] The subunits are linked by a single covalent disulfide bond.
The βC subunit is able to form activin heterodimers with βA or βB subunits but is unable to dimerize with inhibin α.[13]
Function
Activin
Activin is produced in the gonads, pituitary gland, placenta, and other organs:
- In the ovarian follicle, activin increases FSH binding and FSH-induced aromatization. It participates in androgen synthesis enhancing LH action in the ovary and testis. In the male, activin enhances spermatogenesis.
- Activin is strongly expressed in wounded skin, and overexpression of activin in epidermis of transgenic mice improves wound healing and enhances scar formation. Its action in wound repair and skin morphogenesis is through stimulation of keratinocytes and stromal cells in a dose-dependent manner.[14]
- Activin also regulates the morphogenesis of branching organs such as the prostate, lung, and especially kidney. Activin A increased the expression level of type-I collagen suggesting that activin A acts as a potent activator of fibroblasts.
- Lack of activin during development results in neural developmental defects.
- Upregulation of Activin A drives pluripotent stem cells into a mesoendodermal fate, and thus provides a useful tool for stem cell differentiation and organoid formation.[15]
Inhibin
In both females and males, inhibin inhibits FSH production. Inhibin does not inhibit the secretion of GnRH from the hypothalamus.[16][17] However, the overall mechanism differs between the sexes:
In females
Inhibin is produced in the gonads, pituitary gland, placenta, corpus luteum and other organs.
FSH stimulates the secretion of inhibin from the granulosa cells of the ovarian follicles in the ovaries. In turn, inhibin suppresses FSH.
- Inhibin B reaches a peak in the early- to mid-follicular phase, and a second peak at ovulation.
- Inhibin A reaches its peak in the mid-luteal phase.
Inhibin secretion is diminished by GnRH, and enhanced by insulin-like growth factor-1 (IGF-1).
In males
It is secreted from the Sertoli cells,[18] located in the seminiferous tubules inside the testes. Androgens stimulate inhibin production; this protein may also help to locally regulate spermatogenesis.[19]
Mechanism of action
Activin
As with other members of the superfamily, activins interact with two types of cell surface transmembrane receptors (Types I and II) which have intrinsic serine/threonine kinase activities in their cytoplasmic domains:
- Activin type 1 receptors: ACVR1, ACVR1B, ACVR1C
- Activin type 2 receptors: ACVR2A, ACVR2B
Activin binds to the Type II receptor and initiates a cascade reaction that leads to the recruitment, phosphorylation, and activation of Type I activin receptor. This then interacts with and then phosphorylates SMAD2 and SMAD3, two of the cytoplasmic SMAD proteins.
Smad3 then translocates to the nucleus and interacts with SMAD4 through multimerization, resulting in their modulation as transcription factor complexes responsible for the expression of a large variety of genes.
Inhibin
In contrast to activin, much less is known about the mechanism of action of inhibin, but may involve competing with activin for binding to activin receptors and/or binding to inhibin-specific receptors.[8]
Clinical significance
Activin
Activin A is more plentiful in the adipose tissue of obese, compared to lean persons.[20] Activin A promotes the proliferation of adipocyte progenitor cells, while inhibiting their differentiation into adipocytes.[20] Activin A also increases inflammatory cytokines in macrophages.[20]
A mutation in the gene for the activin receptor ACVR1 results in fibrodysplasia ossificans progressiva, a fatal disease that causes muscle and soft tissue to gradually be replaced by bone tissue.[21] This condition is characterized by the formation of an extra skeleton that produces immobilization and eventually death by suffocation.[21] The mutation in ACVR1 causes activin A, which normally acts as an antagonist of the receptor and blocks osteogenesis (bone growth), to behave as an agonist of the receptor and to induce hyperactive bone growth.[21] On 2 September 2015, Regeneron Pharmaceuticals announced that they had developed an antibody for activin A that effectively cures the disease in an animal model of the condition.[22]
Mutations in the ACVR1 gene have also been linked to cancer, especially diffuse intrinsic pontine glioma(DIPG).[23][24][25]
Elevated Activin B levels with normal Activin A levels provided a possible biomarker for myalgic encephalomyelitis/chronic fatigue syndrome.[26]
Activin A is overexpressed in many cancers. It was shown to promote tumorigenesis by hampering the adaptive anti-tumor immune response in melanoma.[27]
Inhibin
Quantification of inhibin A is part of the prenatal quad screen that can be administered during pregnancy at a gestational age of 16–18 weeks. An elevated inhibin A (along with an increased beta-hCG, decreased AFP, and a decreased estriol) is suggestive of the presence of a fetus with Down syndrome.[28] As a screening test, abnormal quad screen test results need to be followed up with more definitive tests.
It also has been used as a marker for ovarian cancer.[29][30]
Inhibin B may be used as a marker of spermatogenesis function and male infertility. The mean serum inhibin B level is significantly higher among fertile men (approximately 140 pg/mL) than in infertile men (approximately 80 pg/mL).[31] In men with azoospermia, a positive test for inhibin B slightly raises the chances for successfully achieving pregnancy through testicular sperm extraction (TESE), although the association is not very substantial, having a sensitivity of 0.65 (95% confidence interval [CI]: 0.56–0.74) and a specificity of 0.83 (CI: 0.64–0.93) for prediction the presence of sperm in the testes in non-obstructive azoospermia.[32]
References
- ↑ Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D, Spiess J (1986). "Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid". Nature. 321 (6072): 776–9. Bibcode:1986Natur.321..776V. doi:10.1038/321776a0. PMID 3012369. S2CID 4365045.
- ↑ Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R (1986). "Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin". Nature. 321 (6072): 779–82. Bibcode:1986Natur.321..779L. doi:10.1038/321779a0. PMID 3086749. S2CID 38100413.
- ↑ Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Chung J, Ying SY (May 2006). "Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis". Experimental Biology and Medicine. 231 (5): 534–44. doi:10.1177/153537020623100507. PMID 16636301. S2CID 39050907.
- ↑ Sulyok S, Wankell M, Alzheimer C, Werner S (October 2004). "Activin: an important regulator of wound repair, fibrosis, and neuroprotection". Molecular and Cellular Endocrinology. 225 (1–2): 127–32. doi:10.1016/j.mce.2004.07.011. PMID 15451577. S2CID 6943949.
- ↑ van Zonneveld P, Scheffer GJ, Broekmans FJ, Blankenstein MA, de Jong FH, Looman CW, Habbema JD, te Velde ER (March 2003). "Do cycle disturbances explain the age-related decline of female fertility? Cycle characteristics of women aged over 40 years compared with a reference population of young women". Human Reproduction. 18 (3): 495–501. doi:10.1093/humrep/deg138. PMID 12615813.
- ↑ Makanji Y, Zhu J, Mishra R, Holmquist C, Wong WP, Schwartz NB, Mayo KE, Woodruff TK (October 2014). "Inhibin at 90: from discovery to clinical application, a historical review". Endocrine Reviews. 35 (5): 747–94. doi:10.1210/er.2014-1003. PMC 4167436. PMID 25051334.
- 1 2 Burger HG, Igarashi M (April 1988). "Inhibin: definition and nomenclature, including related substances". The Journal of Clinical Endocrinology and Metabolism. 66 (4): 885–6. PMID 3346366.
- 1 2 Robertson DM, Burger HG, Fuller PJ (March 2004). "Inhibin/activin and ovarian cancer". Endocrine-Related Cancer. 11 (1): 35–49. doi:10.1677/erc.0.0110035. PMID 15027884. S2CID 12202820.
- 1 2 Kingsley DM (January 1994). "The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms". Genes & Development. 8 (2): 133–46. doi:10.1101/gad.8.2.133. PMID 8299934.
- ↑ Ying SY (December 1987). "Inhibins and activins: chemical properties and biological activity". Proceedings of the Society for Experimental Biology and Medicine. 186 (3): 253–64. doi:10.3181/00379727-186-42611a. PMID 3122219. S2CID 36872324.
- ↑ Xu P, Hall AK (November 2006). "The role of activin in neuropeptide induction and pain sensation". Developmental Biology. 299 (2): 303–9. doi:10.1016/j.ydbio.2006.08.026. PMID 16973148.
- ↑ Deli A, Kreidl E, Santifaller S, Trotter B, Seir K, Berger W, Schulte-Hermann R, Rodgarkia-Dara C, Grusch M (March 2008). "Activins and activin antagonists in hepatocellular carcinoma". World Journal of Gastroenterology. 14 (11): 1699–709. doi:10.3748/wjg.14.1699. PMC 2695910. PMID 18350601.
- ↑ Mellor SL, Cranfield M, Ries R, Pedersen J, Cancilla B, de Kretser D, Groome NP, Mason AJ, Risbridger GP (December 2000). "Localization of activin beta(A)-, beta(B)-, and beta(C)-subunits in humanprostate and evidence for formation of new activin heterodimers of beta(C)-subunit". The Journal of Clinical Endocrinology and Metabolism. 85 (12): 4851–8. doi:10.1210/jc.85.12.4851. PMID 11134153.
- ↑ Bamberger, Casimir; Schärer, Agnes; Antsiferova, Maria; Tychsen, Birte; Pankow, Sandra; Müller, Mischa; Rülicke, Thomas; Paus, Ralf; Werner, Sabine (9 March 2021). "Activin Controls Skin Morphogenesis and Wound Repair Predominantly via Stromal Cells and in a Concentration-Dependent Manner via Keratinocytes". The American Journal of Pathology. 167 (3): 733–747. doi:10.1016/S0002-9440(10)62047-0. PMC 1698729. PMID 16127153.
- ↑ Pauklin S, Vallier L (2015). "Activin/Nodal signalling in stem cells". Development. 142 (4): 607–19. doi:10.1242/dev.091769. PMID 25670788.
- ↑ Luisi S, Florio P, Reis FM, Petraglia F (2005). "Inhibins in female and male reproductive physiology: role in gametogenesis, conception, implantation and early pregnancy". Human Reproduction Update. 11 (2): 123–35. doi:10.1093/humupd/dmh057. PMID 15618291.
- ↑ Le T, Bhushan V, Hofmann J (2012). First Aid for the USMLE Step 1. McGraw Hill. p. 534. ISBN 978-0-07-177636-3.
- ↑ Skinner MK, McLachlan RI, Bremner WJ (October 1989). "Stimulation of Sertoli cell inhibin secretion by the testicular paracrine factor PModS". Molecular and Cellular Endocrinology. 66 (2): 239–49. doi:10.1016/0303-7207(89)90036-1. hdl:1773/4395. PMID 2515083. S2CID 21885326.
- ↑ Meachem SJ, Nieschlag E, Simoni M (November 2001). "Inhibin B in male reproduction: pathophysiology and clinical relevance". European Journal of Endocrinology. 145 (5): 561–71. doi:10.1530/eje.0.1450561. PMID 11720872.
- 1 2 3 Zaragosi LE, Wdziekonski B, Villageois P, Keophiphath M, Maumus M, Tchkonia T, Bourlier V, Mohsen-Kanson T, Ladoux A, Elabd C, Scheideler M, Trajanoski Z, Takashima Y, Amri EZ, Lacasa D, Sengenes C, Ailhaud G, Clément K, Bouloumie A, Kirkland JL, Dani C (2010). "Activin a plays a critical role in proliferation and differentiation of human adipose progenitors". Diabetes. 59 (10): 2513–2521. doi:10.2337/db10-0013. PMC 3279533. PMID 20530742.
- 1 2 3 Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS (May 2006). "A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva". Nature Genetics. 38 (5): 525–527. doi:10.1038/ng1783. PMID 16642017. S2CID 41579747.
- ↑ Julie Steenhuysen (2 September 2015). "Regeneron scientists discover key to excess bone growth in rare disease". Reuters.
- ↑ Taylor KR, Mackay A, Truffaux N, Butterfield YS, Morozova O, Philippe C, Castel D, Grasso CS, Vinci M, Carvalho D, Carcaboso AM, de Torres C, Cruz O, Mora J, Entz-Werle N, Ingram WJ, Monje M, Hargrave D, Bullock AN, Puget S, Yip S, Jones C, Grill J (May 2014). "Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma". Nature Genetics. 46 (5): 457–61. doi:10.1038/ng.2925. PMC 4018681. PMID 24705252.
- ↑ "Cure Brain Cancer - News - Multiple Breakthroughs in Childhood Brain Cancer DIPG". Cure Brain Cancer Foundation.
- ↑ Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, et al. (May 2014). "Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations". Nature Genetics. 46 (5): 451–6. doi:10.1038/ng.2936. PMC 3997489. PMID 24705254.
- ↑ Lidbury BA, Kita B, Lewis DP, Hayward S, Ludlow H, Hedger MP, de Kretser DM (March 2017). "Activin B is a novel biomarker for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) diagnosis: a cross sectional study". Journal of Translational Medicine. 15 (1): 60. doi:10.1186/s12967-017-1161-4. PMC 5353946. PMID 28302133.
- ↑ Donovan P, Dubey OA, Kallioinen S, Rogers KW, Muehlethaler K, Müller P, et al. (December 2017). "Paracrine Activin-A Signaling Promotes Melanoma Growth and Metastasis through Immune Evasion". The Journal of Investigative Dermatology. 137 (12): 2578–2587. doi:10.1016/j.jid.2017.07.845. PMID 28844941.
- ↑ Aitken DA, Wallace EM, Crossley JA, Swanston IA, van Pareren Y, van Maarle M, Groome NP, Macri JN, Connor JM (May 1996). "Dimeric inhibin A as a marker for Down's syndrome in early pregnancy". The New England Journal of Medicine. 334 (19): 1231–6. doi:10.1056/NEJM199605093341904. PMID 8606718.
- ↑ Robertson DM, Pruysers E, Jobling T (April 2007). "Inhibin as a diagnostic marker for ovarian cancer". Cancer Letters. 249 (1): 14–7. doi:10.1016/j.canlet.2006.12.017. PMID 17320281.
- ↑ Robertson DM, Pruysers E, Burger HG, Jobling T, McNeilage J, Healy D (October 2004). "Inhibins and ovarian cancer". Molecular and Cellular Endocrinology. 225 (1–2): 65–71. doi:10.1016/j.mce.2004.02.014. PMID 15451569. S2CID 33801243.
- ↑ Myers GM, Lambert-Messerlian GM, Sigman M (December 2009). "Inhibin B reference data for fertile and infertile men in Northeast America". Fertility and Sterility. 92 (6): 1920–3. doi:10.1016/j.fertnstert.2008.09.033. PMID 19006797.
- ↑ Toulis KA, Iliadou PK, Venetis CA, Tsametis C, Tarlatzis BC, Papadimas I, Goulis DG (2010). "Inhibin B and anti-Mullerian hormone as markers of persistent spermatogenesis in men with non-obstructive azoospermia: a meta-analysis of diagnostic accuracy studies". Human Reproduction Update. 16 (6): 713–24. doi:10.1093/humupd/dmq024. PMID 20601364.
External links
- Activin at the US National Library of Medicine Medical Subject Headings (MeSH)
- Inhibin at the US National Library of Medicine Medical Subject Headings (MeSH)
- Grusch M, Kreidl E (1 August 2008). "Activin and follistatin in liver biology and hepatocellular carcinoma". SciTopics. Elsevier. Archived from the original on 9 December 2008. Retrieved 24 December 2008.