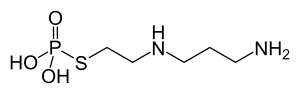
Amifostine
 | |
| Names | |
|---|---|
| Other names | Ethiofos |
IUPAC name
| |
| Clinical data | |
| Main uses | Prevent toxicity from chemotherapy or radiotherapy[1] |
| Side effects | Low blood pressure, nausea[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | complete |
| Elimination half-life | 8 minutes |
| Chemical and physical data | |
| Formula | C5H15N2O3PS |
| Molar mass | 214.22 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Amifostine, sold under the brand name Ethyol, is a medication used to prevent toxicity related to chemotherapy and radiotherapy.[1] Specifically it is used to prevent kidney toxicity from cisplatin and parotid gland damage from head and neck radiation.[1] It is given by injection into a vein.[1]
Common side effects include low blood pressure and nausea.[1] Other side effects may include severe skin rashes, allergic reactions, and low calcium.[1] Use during pregnancy may harm the baby.[1] It is a cytoprotective agent.[2]
Amifostine was approved for medical use in the United States in 1995.[1] In the United States a 500 mg vial costs about 480 USD as of 2022.[3]
Medical uses
Amifostine is used therapeutically to reduce the incidence of neutropenia-related fever and infection induced by DNA-binding chemotherapeutic agents including alkylating agents (e.g. cyclophosphamide) and platinum-containing agents (e.g. cisplatin). It is also used to decrease the cumulative nephrotoxicity associated with platinum-containing agents. Amifostine is also indicated to reduce the incidence of xerostomia in patients undergoing radiotherapy for head and neck cancer.
Amifostine was originally indicated to reduce the cumulative renal toxicity from cisplatin in non-small cell lung cancer. However, while nephroprotection was observed, the probability that amifostine could protect tumors could not be excluded. Additional data have shown that amifostine-mediated tumor protection, in any clinical scenario, is unlikely.
Side effects
Common side effects include hypocalcemia, diarrhea, nausea, vomiting, sneezing, somnolence, and hiccoughs. Serious side effects include: hypotension (found in 62% of patients), erythema multiforme, Stevens–Johnson syndrome and toxic epidermal necrolysis, immune hypersensitivity syndrome, erythroderma, anaphylaxis, and loss of consciousness (rare).
Contraindications
Contraindications to receiving amifostine include hypersensitivity to amifostine and aminothiol compounds like WR-1065. Ethyol contains mannitol.
Pharmacokinetics
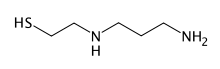
Amifostine is an organic thiophosphate prodrug which is hydrolysed in vivo by alkaline phosphatase to the active cytoprotective thiol metabolite, WR-1065. The selective protection of non-malignant tissues is believed to be due to higher alkaline phosphatase activity, higher pH, and vascular permeation of normal tissues.
Amifostine can be administered intravenously or subcutaneously after reconstitution with normal saline. Infusions lasting less than 15 minutes decrease the risk of adverse effects. The patient should be well-hydrated prior to administration.
Mechanism of action
Inside cells, amifostine detoxifies reactive metabolites of platinum and alkylating agents, as well as scavenges free radicals.[4][5] Other possible effects include accelerated DNA repair,[4] induction of cellular hypoxia,[4] inhibition of apoptosis,[5] alteration of gene expression[5] and modification of enzyme activity.[5] Amifostine is believed to radioprotect normal tissue via Warburg-type effects.[6]
References
- 1 2 3 4 5 6 7 8 9 "DailyMed - ETHYOL- amifostine injection, powder, lyophilized, for solution". dailymed.nlm.nih.gov. Archived from the original on 25 March 2021. Retrieved 14 January 2022.
- ↑ "Amifostine Monograph for Professionals". Drugs.com. Archived from the original on 7 April 2021. Retrieved 14 January 2022.
- ↑ "Amifostine Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 14 January 2022.
- 1 2 3 Kouvaris JR, Kouloulias VE, Vlahos LJ (June 2007). "Amifostine: the first selective-target and broad-spectrum radioprotector". Oncologist. 12 (6): 738–47. doi:10.1634/theoncologist.12-6-738. PMID 17602063.
- 1 2 3 4 "Amifostine : BC Cancer Agency". British Columbia Cancer Agency. 2006-03-01. Archived from the original on 2015-03-14. Retrieved 2011-01-01.
- ↑ Koukourakis, Michael I.; Giatromanolaki, Alexandra; Zois, Christos E.; Kalamida, Dimitra; Pouliliou, Stamatia; Karagounis, Ilias V.; Yeh, Tzu-Lan; Abboud, Martine I.; Claridge, Timothy D. W. (2016-08-10). "Normal tissue radioprotection by amifostine via Warburg-type effects". Scientific Reports. 6: 30986. Bibcode:2016NatSR...630986K. doi:10.1038/srep30986. ISSN 2045-2322. PMC 4978965. PMID 27507219.
External links
| Identifiers: |
|---|