Amikhelline
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Metabolism | none |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
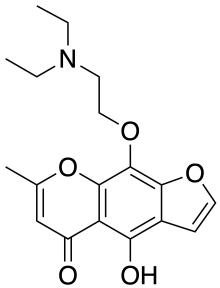
| Formula | C18H21NO5 |
| Molar mass | 331.368 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Amikhelline is an antimitotic drug.[1] It acts as a DNA intercalator[2] and inhibits DNA polymerase.[3]
References
- ↑ Turchini MF, Geneix A, Perissel B, Malet P, Turchini JP (1985). "[Characterization of chromosomal aberrations induced in man by various antimitotic agents]". Comptes Rendus des Séances de la Société de Biologie et de ses Filiales. 179 (3): 331–9. PMID 2417673.
- ↑ Rucheton M, Jeanteur P (1973). "Studies on amikhellin. I. Intercalative binding to double-stranded DNA". Biochimie. 55 (11): 1415–20. doi:10.1016/s0300-9084(74)80548-1. PMID 4364376.
- ↑ Rucheton M, Jeanteur P (1976). "Studies on amikhellin. II.-Inhibition of dna-polymerase from murine sarcoma leukemia virus". Biochimie. 58 (6): 689–95. doi:10.1016/s0300-9084(76)80393-8. PMID 60141.
]
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.