Anterior cervical discectomy and fusion
| Anterior cervical discectomy and fusion | |
|---|---|
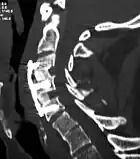
 X-rays of anterior cervical discectomy and fusion, C5C6 and C6C7. Lateral view. | |
| Specialty | neurosurgeon |
Anterior cervical discectomy and fusion (ACDF) is a surgical procedure to treat nerve root or spinal cord compression by decompressing the spinal cord and nerve roots of the cervical spine with a discectomy, followed by inter-vertebral fusion to stabilize the corresponding vertebrae.[1] This procedure is used when other non-surgical treatments have failed.
Medical uses
ACDF is used to treat serious pain from a nerve root that has become inflamed. This can be caused by:
1. a herniated disc when other non-surgical treatments have failed. The nucleus pulposus (the jelly-like center of the disc) of the herniated disc bulges out through the annulus (surrounding wall) and presses on the nerve root next to it.
2. degenerative disc disease (spondylosis). The disc consists of about 80% water. When one grows older, the disc starts to dry out and shrink, causing small tears in the annulus and inflammation of the nerve root.
Contraindications
Bone morphogenetic protein (rhBMP) should not be routinely used in any type of anterior cervical spine fusion, such as with anterior cervical discectomy and fusion.[2][3] There are reports of this therapy causing swelling of soft tissue which in turn can cause life-threatening complications due to difficulty swallowing and pressure on the respiratory tract.[1][3]
Technique

The neurosurgeon or orthopedic surgeon enters the space between two discs through a small incision in front (= anterior) of and at the right or left side of the neck. The disc is completely removed, as well as arthritic bone spurs. The disc material, pressing on the spinal nerve or spinal cord, is then completely removed. The intervertebral foramen, the bone channel through which the spinal nerve runs, is then enlarged with a drill giving the nerve more room to exit the spinal canal.
To prevent the vertebrae from collapsing and to increase stability, the open space is often filled with a graft. That can be a bone graft, taken from the pelvis or cadaveric bone; or an artificial implant.[4] The slow process of the bone graft joining the vertebrae together is called "fusion". Sometimes a titanium plate is screwed on the vertebrae or screws are used between the vertebrae to increase stability during fusion, especially when there is more than one disc involved.
Recovery
The surgery requires a short stay in the clinic (1 to 3 days) and a gradual recovery between 1 and 6 weeks. However, the technology has advanced and it can be performed by 'Endoscopic Micro Discectomy" with the patient able to continue their normal life in two days. The patient may be advised to wear a neck brace or collar (for up to 8 weeks) that serves to ensure proper spinal alignment. Wearing the brace heightens one's awareness of posture and positioning and helps prevent movements (e.g., sudden and/or excessive bending or twisting of the neck) that may aggravate or slow down the healing process.
It is especially advisable to wear a protective neck brace when traveling (e.g., by car), sleeping, showering, or any other activities in which the patient may not be able to be ensure proper spinal alignment. In addition, physical therapy and related healing modalities (e.g., massage, acupuncture) may be recommended in order to promote proper healing, as well as to strengthen the surrounding muscles that can take over the neck brace's 'job' of ensuring proper spinal alignment when the patient starts (around 4 to 6 weeks after surgery) to wean off the neck brace.
References
- 1 2 Zadegan SA, Jazayeri SB, Abedi A, Bonaki HN, Vaccaro AR, Rahimi-Movaghar V (May 2018). "Corticosteroid Administration to Prevent Complications of Anterior Cervical Spine Fusion: A Systematic Review". Global Spine Journal. 8 (3): 286–302. doi:10.1177/2192568217708776. PMC 5958478. PMID 29796378.
- ↑ Zadegan SA, Abedi A, Jazayeri SB, Nasiri Bonaki H, Jazayeri SB, Vaccaro AR, Rahimi-Movaghar V (August 2017). "Bone Morphogenetic Proteins in Anterior Cervical Fusion: A Systematic Review and Meta-Analysis". World Neurosurgery. 104: 752–787. doi:10.1016/j.wneu.2017.02.098. PMID 28315798.
- 1 2 North American Spine Society (February 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, North American Spine Society, retrieved 25 March 2013, which cites
- Schultz, Daniel G. (July 1, 2008). "Public Health Notifications (Medical Devices) - FDA Public Health Notification: Life-threatening Complications Associated with Recombinant Human Bone Morphogenetic Protein in Cervical Spine Fusion". fda.gov. Retrieved 25 March 2014.
- Woo EJ (October 2012). "Recombinant human bone morphogenetic protein-2: adverse events reported to the Manufacturer and User Facility Device Experience database". The Spine Journal. 12 (10): 894–9. doi:10.1016/j.spinee.2012.09.052. PMID 23098616.
- ↑ Zadegan SA, Abedi A, Jazayeri SB, Bonaki HN, Vaccaro AR, Rahimi-Movaghar V (June 2017). "Clinical Application of Ceramics in Anterior Cervical Discectomy and Fusion: A Review and Update". Global Spine Journal. 7 (4): 343–349. doi:10.1177/2192568217699201. PMC 5546682. PMID 28815162.