Azamulin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
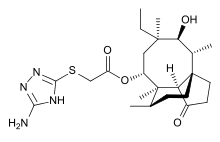
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H38N4O4S |
| Molar mass | 478.65 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Azamulin is a pleuromutilin antibiotic.[1] As of 2021, it is not marketed in the US or Europe.
In pharmacological studies, the substance is used as an inhibitor of the liver enzymes CYP3A4 and CYP3A5.[2][3]
References
- ↑ Stresser DM, Broudy MI, Ho T, Cargill CE, Blanchard AP, Sharma R, et al. (January 2004). "Highly selective inhibition of human CYP3Aa in vitro by azamulin and evidence that inhibition is irreversible". Drug Metabolism and Disposition. 32 (1): 105–12. doi:10.1124/dmd.32.1.105. PMID 14709627.
- ↑ Ghosal A, Ramanathan R, Yuan Y, Hapangama N, Chowdhury SK, Kishnani NS, Alton KB (December 2007). "Identification of human liver cytochrome P450 enzymes involved in biotransformation of vicriviroc, a CCR5 receptor antagonist". Drug Metabolism and Disposition. 35 (12): 2186–95. doi:10.1124/dmd.107.017517. PMID 17827338. S2CID 1866233.
- ↑ Mitra R, Goodman OB (April 2015). "CYP3A5 regulates prostate cancer cell growth by facilitating nuclear translocation of AR". The Prostate. 75 (5): 527–38. doi:10.1002/pros.22940. PMID 25586052. S2CID 19910736.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.