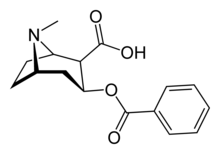
Benzoylecgonine
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1R,2R,3S,5S)-3-(Benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
89637 |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.513 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C16H19NO4 |
| Molar mass | 289.331 g·mol−1 |
| Hazards | |
| GHS labelling: | |
Pictograms |
 |
Signal word |
Danger |
Hazard statements |
H301 |
Precautionary statements |
P264, P270, P301+P310, P321, P330, P405, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzoylecgonine is the main metabolite of cocaine, formed by the liver and excreted in the urine. It is the compound tested for in most cocaine urine drug screens.
Pharmacokinetics
Chemically, benzoylecgonine is the benzoate ester of ecgonine. It is a primary metabolite of cocaine,[1] and is pharmacologically inactive.[2]
Urinalysis
Benzoylecgonine is the compound tested for in most substantive cocaine drug urinalyses. It is the corresponding carboxylic acid of cocaine, its methyl ester. It is formed in the liver by the metabolism of cocaine by hydrolysis, catalysed by carboxylesterases, and subsequently excreted in the urine. It can be found in the urine for considerably longer than the cocaine itself which is generally cleared out within 5 days.
Presence in drinking water
Benzoylecgonine is sometimes found in drinking water supplies. In 2005, scientists found surprisingly large quantities of benzoylecgonine in Italy's Po River and used its concentration to estimate the number of cocaine users in the region.[3] In 2006, a similar study was performed in the Swiss ski town of Saint-Moritz using wastewater to estimate the daily cocaine consumption of the population.[4] A study done in the United Kingdom found traces of benzoylecgonine in the country's drinking water supply, along with carbamazepine (an anticonvulsant) and ibuprofen (a common non-steroidal anti-inflammatory drug), although the study noted that the amount of each compound present was several orders of magnitude lower than the therapeutic dose and therefore did not pose a risk to the population.[5]
Preliminary studies on ecological systems show that benzoylecgonine has potential toxicity issues.[6] Research is being conducted on degradation options such as advanced oxidation and photocatalysis[7] for this metabolite in an effort to reduce concentrations in waste and surface waters. At environmentally relevant concentrations, benzoylecgonine has been shown to have a negative ecological impact.[6]
See also
- Coca alkaloids
- Dihydrocuscohygrine
- Hygrine
References
- ↑ Schindler, Charles W; Goldberg, Steven R (2012). "Accelerating cocaine metabolism as an approach to the treatment of cocaine abuse and toxicity". Future Medicinal Chemistry. 4 (2): 163–75. doi:10.4155/fmc.11.181. PMC 3293209. PMID 22300096.
- ↑ Shimomura, Eric T.; Jackson, George F.; Paul, Buddha Dev (2019-01-01), Dasgupta, Amitava (ed.), "Chapter 17 - Cocaine, Crack Cocaine, and Ethanol: A Deadly Mix", Critical Issues in Alcohol and Drugs of Abuse Testing (Second Edition), Academic Press, pp. 215–224, doi:10.1016/b978-0-12-815607-0.00017-4, ISBN 978-0-12-815607-0, retrieved 2020-12-23
- ↑ "Italian river 'full of cocaine'". BBC News. 5 August 2005. Retrieved 11 May 2014.
- ↑ "Tant de coke ? Stupéfiant !". Courrier International (in French). 2 February 2006. Retrieved 11 May 2014.
- ↑ Withnall, Adam (11 May 2014). "Cocaine use in Britain so high it has contaminated our drinking water, report shows". The Independent. Retrieved 11 May 2014.
- 1 2 Binelli, A.; Marisa, I; Fedorova, M; Hoffmann, R; Riva, C (2013). "First evidence of protein profile alteration due to the main cocaine metabolite (benzoylecgonine) in a freshwater model". Aquatic Toxicology. 140–141: 268–278. doi:10.1016/j.aquatox.2013.06.013. PMID 23838174.
- ↑ Postigo, C.; Sirtori, C.; Oller, I.; Malato, S.; Maldonado, M.I.; Lopez de Alda, M.; Barcelo, D. (2011). "Solar transformation and photocatalytic treatment of cocaine in water: kinetics, characterization of major intermediate products and toxicity evaluation". Applied Catalysis B: Environmental. 104 (1–2): 37–48. doi:10.1016/j.apcatb.2011.02.030.