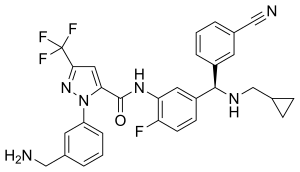
Berotralstat
 | |
| Names | |
|---|---|
| Trade names | Orladeyo |
| Other names | BCX7353, BCX-7353 |
IUPAC name
| |
| Clinical data | |
| Drug class | Plasma kallikrein inhibitor |
| Main uses | Hereditary angioedema (HAE)[1] |
| Side effects | Abdominal pain, vomiting, diarrhea, back pain, heartburn[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | 150 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C30H26F4N6O |
| Molar mass | 562.573 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Berotralstat, sold under the brand name Orladeyo, is a medication used to prevent attacks of hereditary angioedema (HAE).[1] It is used in people aged twelve years and older.[1] It is taken by mouth.[1]
Common side effects include abdominal pain, vomiting, diarrhea, back pain, and heartburn.[1] Other side effects may include QT prolongation.[1] While there is no evidence of harm in pregnancy, such use has not been well studied.[1] It is a plasma kallikrein inhibitor.[1]
Berotralstat was approved for medical use in the United States in 2020 and Europe in 2021.[1][4] In the United Kingdom 4 weeks costs the NHS about £10,200 as of 2022.[5] This amount in the United States costs about 40,500 USD.[6]
Medical uses
It is used to prevent attacks of hereditary angioedema (HAE).[1] It decreases the rate of attacks from about 2.4 per month on placebo to 1.3 with treatment.[4]
Dosage
It is generally taken at a dose of 150 mg once per day.[1]
History
Berotralstat was approved based on evidence from one clinical trial (Trial 1 /NCT03485911) of 120 participants with hereditary angioedema.[3] The trial was conducted at 40 sites in the United States, the European Union, and Canada.[3] Trial investigators evaluated participants 12 years and older[7] with hereditary angioedema for eight weeks to determine the number of attacks for each participant.[3] The trial enrolled only participants who had at least two attacks during the eight-week period.[3] Participants were assigned to receive one of two doses of berotralstat or placebo once every day for 24 weeks.[3] Neither the participants nor the investigators knew which treatment was being given until after the trial was completed.[3] All participants could use other medications for treatment of attacks.[3]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Orladeyo- berotralstat hydrochloride capsule". DailyMed. Archived from the original on 1 November 2022. Retrieved 25 December 2020.
- ↑ https://pdf.hres.ca/dpd_pm/00066149.PDF Archived 1 October 2022 at the Wayback Machine
- 1 2 3 4 5 6 7 8 "Drug Trials Snapshot: Orladeyo". U.S. Food and Drug Administration. 3 December 2020. Archived from the original on 24 October 2021. Retrieved 25 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 "Orladeyo EPAR". European Medicines Agency (EMA). 24 February 2021. Archived from the original on 12 July 2021. Retrieved 12 July 2021.
- ↑ "Berotralstat". SPS - Specialist Pharmacy Service. 21 July 2018. Archived from the original on 12 December 2021. Retrieved 3 November 2022.
- ↑ "Orladeyo Prices, Coupons, Copay & Patient Assistance". Drugs.com. Archived from the original on 21 May 2022. Retrieved 3 November 2022.
- ↑ "Berotralstat (Oral Route) Side Effects - Mayo Clinic". www.mayoclinic.org. Archived from the original on 17 April 2021. Retrieved 3 March 2021.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Clinical trial number NCT03485911 for "Efficacy and Safety Study of BCX7353 as an Oral Treatment for the Prevention of Attacks in HAE (APeX-2)" at ClinicalTrials.gov