Choanozoa
| Choanozoa Temporal range: Molecular clock evidence for origin between 1050 and 800Ma[1] | |
|---|---|
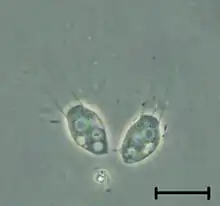 | |
| Codosiga | |
| Scientific classification | |
| (unranked): | Unikonta |
| (unranked): | Obazoa |
| (unranked): | Opisthokonta |
| (unranked): | Holozoa |
| (unranked): | Filozoa |
| (unranked): | Choanozoa Brunet and King, 2017 |
| Subdivisions | |
| |
| Synonyms | |
|
Apoikozoa Budd & Jensen, 2015 Choanimal Fairclough et al., 2013 Choanoflagellates | |
Choanozoa is a clade of opisthokont eukaryotes consisting of the choanoflagellates (Choanoflagellatea) and the animals (Animalia, Metazoa). The sister-group relationship between the choanoflagellates and animals has important implications for the origin of the animals.[2] The clade was identified in 2015 by Graham Budd and Sören Jensen, who used the name Apoikozoa.[3] The 2018 revision of the classification first proposed by the International Society of Protistologists in 2012 recommends the use of the name Choanozoa.[4]
Introduction
A close relationship between choanoflagellates and animals has long been recognised, dating back at least to the 1840s. A particularly striking and famous similarity between the single-celled choanoflagellates and multicellular animals is provided by the collar cells of sponges and the overall morphology of the choanoflagellate cell. The relationship has since been confirmed by multiple molecular analyses. This proposed homology was however thrown into some doubt in 2013 by the still controversial suggestion that ctenophores, and not sponges, are the sister group to all other animals.[5][6] More recent genomic work has suggested that choanoflagellates possess some of the important genetic machinery necessary for the multicellularity found in animals.
A synonym for the Choanozoa, Apoikozoa, derives from the ancient Greek for "colony" and "animal", referring to the ability of both animals and (some) choanoflagellates to form multicellular units.[4] While animals are permanently multicellular, the colony-building choanoflagellates are only sometimes so, which raises the question of whether or not the colony-building ability in both groups was present at the base of the entire clade, or whether it was independently derived within the animals and choanoflagellates. In either case, these two groups are the only heterotrophs known to form colonies.
Nomenclature
The name "Choanozoa" was used by Thomas Cavalier-Smith in 1991 to refer to a group of basal protists that later proved not to form a clade. Adl et al. (2018) regard the name as appropriate for the clade of choanoflagellates and animals, since the Greek choanē (χοάνη), meaning 'funnel', refers to the collar, which is a synapomorphy of the clade. They reject the name "Apoikozoa" as being neither formally defined nor appropriate, since it refers to the ability to form colonies, which is not unique to this clade.[4]
Evolutionary implications
Although the last common ancestor of the Choanozoa cannot be reconstructed with certainty, Budd and Jensen suggest that these organisms formed benthic colonies that competed for space amongst other mat-forming organisms known to have existed during the Ediacaran Period some 635–540 million years ago. As such they would form an important link between the unicellular ancestors of the animals and the enigmatic "Ediacaran" organisms known from this interval, thus allowing some sort of reconstruction of the earliest animals and their ecology.[3] In the following cladogram, an indication is given of approximately how many million years ago (Mya) the clades diverged into newer clades.[7][8][9] (Note that the later Budd and Jensen paper gives significantly younger dates. See also Kimberella.) The holomycota tree follows Tedersoo et al.[10]
| Opisthokonta |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1300 mya |
References
- ↑ Laura Wegener Parfrey; Daniel J G Lahr; Andrew H Knoll; Laura A Katz (16 August 2011). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 108 (33): 13624–9. Bibcode:2011PNAS..10813624P. doi:10.1073/PNAS.1110633108. ISSN 0027-8424. PMC 3158185. PMID 21810989. Wikidata Q24614721.
- ↑ King, N.; Westbrook, M. J.; Young, S. L.; Kuo, A.; Abedin, M.; Chapman, J.; Fairclough, S.; Hellsten, U.; Isogai, Y.; Letunic, I. (February 14, 2008). "The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans". Nature. 451 (7180): 783–788. Bibcode:2008Natur.451..783K. doi:10.1038/nature06617. PMC 2562698. PMID 18273011.
- 1 2 Budd, G. E.; Jensen, S. (2015). "The origin of the animals and a 'Savannah' hypothesis for early bilaterian evolution". Biological Reviews. 92 (1): 446–473. doi:10.1111/brv.12239. PMID 26588818.
- 1 2 3 Adl, Sina M.; Bass, David; Lane, Christopher E.; Lukeš, Julius; Schoch, Conrad L.; Smirnov, Alexey; Agatha, Sabine; Berney, Cedric; Brown, Matthew W. (2018-09-26). "Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes". Journal of Eukaryotic Microbiology. 66 (1): 4–119. doi:10.1111/jeu.12691. PMC 6492006. PMID 30257078.
- ↑ Ryan, J. F. (December 13, 2013). "The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution". Science. 342 (6164): 1242592. doi:10.1126/science.1242592. PMC 3920664. PMID 24337300.
- ↑ Pisani, D.; Pett, W.; Dohrmann, M.; Feuda, R.; Rota-Stabelli, O.; Philippe, H.; Lartillot, N. & Wörheide, G. (December 15, 2015). "Genomic data do not support comb jellies as the sister group to all other animals". Proceedings of the National Academy of Sciences. 112 (50): 15402–7. Bibcode:2015PNAS..11215402P. doi:10.1073/pnas.1518127112. PMC 4687580. PMID 26621703.
- ↑ Peterson, Kevin J.; Cotton, James A.; Gehling, James G.; Pisani, Davide (2008-04-27). "The Ediacaran emergence of bilaterians: congruence between the genetic and the geological fossil records". Philosophical Transactions of the Royal Society of London B: Biological Sciences. 363 (1496): 1435–1443. doi:10.1098/rstb.2007.2233. PMC 2614224. PMID 18192191.
- ↑ Parfrey, Laura Wegener; Lahr, Daniel J. G.; Knoll, Andrew H.; Katz, Laura A. (2011-08-16). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks". Proceedings of the National Academy of Sciences. 108 (33): 13624–13629. Bibcode:2011PNAS..10813624P. doi:10.1073/pnas.1110633108. PMC 3158185. PMID 21810989.
- ↑ Hehenberger, Elisabeth; Tikhonenkov, Denis V.; Kolisko, Martin; Campo, Javier del; Esaulov, Anton S.; Mylnikov, Alexander P.; Keeling, Patrick J. (2017). "Novel Predators Reshape Holozoan Phylogeny and Reveal the Presence of a Two-Component Signaling System in the Ancestor of Animals". Current Biology. 27 (13): 2043–2050.e6. doi:10.1016/j.cub.2017.06.006. PMID 28648822.
- ↑ Tedersoo, Leho; Sánchez-Ramírez, Santiago; Kõljalg, Urmas; Bahram, Mohammad; Döring, Markus; Schigel, Dmitry; May, Tom; Ryberg, Martin; Abarenkov, Kessy (2018). "High-level classification of the Fungi and a tool for evolutionary ecological analyses". Fungal Diversity. 90 (1): 135–159. doi:10.1007/s13225-018-0401-0. ISSN 1560-2745.
.jpg.webp)