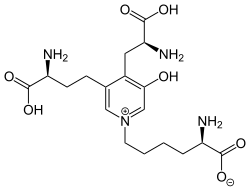
Deoxypyridinoline
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2R)-6-{3-[(3S)-3-Amino-4-hydroxy-4-oxobutyl]-4-[(2S)-2-amino-3-hydroxy-3-oxopropyl]-5-hydroxypyridin-1-ium-1-yl}hexanoate | |
| Identifiers | |
CAS Number |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
Chemical formula |
C18H28N4O7 |
| Molar mass | 412.43752 g mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Deoxypyridinoline, also called D-Pyrilinks, Pyrilinks-D, or deoxyPYD, is one of two pyridinium cross-links that provide structural stiffness to type I collagen found in bones.[1] It is excreted unmetabolized in urine and is a specific marker of bone resorption and osteoclastic activity. It is measured in urine tests and is used along with other bone markers such as alkaline phosphatase, osteocalcin, and N-terminal telopeptide to diagnose bone diseases such as postmenopausal osteoporosis, bone metastasis, and Paget's disease, furthermore, it has been useful in monitoring treatments that contain bone-active agents such as estrogens and bisphosphonates. [1]
Certain studies have attempted to generate a standardization of Deoxypyridinoline via an individual molar absorptivity value at acid and neutrality pH. The result was 5160 and 5290 L mol−1 cm−1 respectively.[2]
References
- 1 2 Rubinacci, A; Melzi, R; Zampino, M; Soldarini, A; Villa, I (1999). "Total and free deoxypyridinoline after acute osteoclast activity inhibition". Clinical Chemistry. 45 (9): 1510–6. doi:10.1093/clinchem/45.9.1510. PMID 10471654.
- ↑ Robins, SP; Duncan, A; Wilson, N; Evans, BJ (1996). "Standardization of pyridinium crosslinks, pyridinoline and deoxypyridinoline, for use as biochemical markers of collagen degradation". Clinical Chemistry. 42 (10): 1621–6. doi:10.1093/clinchem/42.10.1621. PMID 8855145.