Diffuse intrinsic pontine glioma
.png.webp)
A diffuse intrinsic pontine glioma (DIPG) is a tumour located in the pons (middle) of the brain stem. Glioma is a general name for any tumour that arises from the supportive tissue called glia, which help keep the neurons in place and functioning well. DIPG is a brainstem glioma. The brain stem is the bottommost portion of the brain, connecting the cerebrum with the spinal cord. The majority of brain stem tumours occur in the pons and are diffusely infiltrating (they grow amidst the nerves), and therefore cannot be surgically removed. The brain stem contains all of the incoming neurons within the spinal cord, as well as important structures involved in eye movements and in face and throat muscle control and sensation.[1][2]
Diagnosis
.png.webp)
Like most brainstem tumors, diagnosing diffuse intrinsic pontine glioma usually involves non-invasive brain imaging like MRI, in addition to neurologic physical exam. Biopsies and other procedures are very uncommon. Similar to DIPG, diffuse midline gliomas (DMG) often fall into similar categories for both diagnosis and treatment as DIPG and are often categorized together.[3] More recently, biopsies are performed so that the best option for clinical trials can be chosen.
In studies resulting from the DIPG/DMG Registry and in connection with the DIPG/DMG Collaborative, statistics reveal that approximately 150–300 patients are diagnosed with DIPG in the USA per year, the median age of patients with DIPG is approximately 6–7 years old and the male/female ratio of DIPG patients is 1:1.[4]
Treatment
The standard treatment for DIPG is 6 weeks of radiation therapy, which often dramatically improves symptoms. However, symptoms usually recur after 6 to 9 months and progress rapidly.[5]
Neurosurgery
Surgery to attempt tumour removal is usually not possible or advisable for DIPG. By nature, these tumors invade diffusely throughout the brain stem, growing between normal nerve cells. Aggressive surgery would cause severe damage to neural structures vital for arm and leg movement, eye movement, swallowing, breathing, and even consciousness.
A neurosurgically performed brain-stem biopsy for immunotyping of diffuse intrinsic pontine glioma has served a limited recent role in experimental clinical studies and treatment trials. This, however, is not the current standard of care, as it presents considerable risk given the biopsy location, and thus is appropriately performed only in the context of participation in an ongoing clinical treatment trial.
Pontine biopsy is in no way a therapeutic or curative surgery, and the risks (potentially catastrophic and fatal) are only outweighed when the diagnosis is uncertain (extremely unusual) or the patient is enrolled in an approved clinical trial.
Radiotherapy
.png.webp)
Conventional radiotherapy, limited to the involved area of tumour, is the mainstay of treatment for DIPG. A total radiation dosage ranging from 5400 to 6000 cGy, administered in daily fractions of 150 to 200 cGy over 6 weeks, is standard. Hyperfractionated (twice-daily) radiotherapy was used previously to deliver higher radiation dosages, but did not lead to improved survival. Radiosurgery (e.g., gamma knife or cyberknife) has a role in the treatment of DIPG and may be considered in selected cases.
Chemotherapy and other drug therapies
The role of chemotherapy in DIPG remains unclear. Studies have shown little improvement in survival, although efforts (see below) through the Children's Oncology Group (COG), Paediatric Brain Tumour Consortium (PBTC), and others are underway to explore further the use of chemotherapy and other drugs. Drugs that increase the effect of radiotherapy (radiosensitizers) have shown no added benefit, but promising new agents are under investigation. Immunotherapy with beta-interferon and other drugs has also had little effect in trials. Intensive or high-dose chemotherapy with autologous bone marrow transplantation or peripheral blood stem cell rescue has not demonstrated any effectiveness in brain stem gliomas. Future clinical trials may involve medicines designed to interfere with cellular pathways (signal transfer inhibitors), or other approaches that alter the tumor or its environment.[6][7][8]
Prognosis
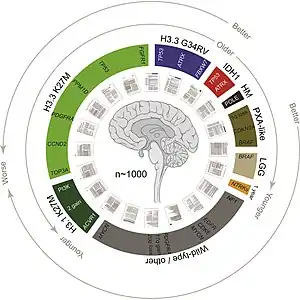
DIPG has a 5-year survival rate of <1%. The median overall survival of children diagnosed with DIPG is approximately 9 months. The 1- and 2-year survival rates are approximately 30% and less than 10%, respectively. These statistics make DIPG one of the most devastating pediatric cancers.[9] Although 75–85% of patients show some improvement in their symptoms after radiation therapy, DIPGs almost always begin to grow again (called recurrence, relapse, or progression). Clinical trials have reported that the median time between radiation therapy and progression is 5–8.8 months.[10] Patients whose tumors begin to grow again may be eligible for experimental treatment through clinical trials to try to slow or stop the growth of the tumor. However, clinical trials have not shown any significant benefit from experimental DIPG therapies so far.[10]
DIPGs that progress usually grow quickly and affect important parts of the brain. The median time from tumor progression to death is usually very short, between 1 and 4.5 months. During this time, doctors focus on palliative care: controlling symptoms and making the patient as comfortable as possible.[10]
Research
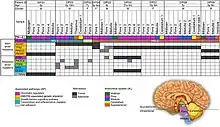
_-_Fphar-08-00495-g004.jpg.webp)
As is the case with most brain tumors, a major difficulty in treating DIPG is overcoming the blood–brain barrier.[11][12]
In the brain – unlike in other areas of the body, where substances can pass freely from the blood into the tissue – there is very little space between the cells lining the blood vessels. Thus, the movement of substances into the brain is significantly limited. This barrier is formed by the lining cells of the vessels as well as by projections from nearby astrocytes. These two types of cells are knitted together by proteins to form what are called "tight junctions". The entire structure is called the blood–brain barrier (BBB). It prevents chemicals, toxins, bacteria, and other substances from getting into the brain, and thus serves a continuous protective function. However, with diseases such as brain tumors, the BBB can also prevent diagnostic and therapeutic agents from reaching their target.
Researchers and clinicians have tried several methods to overcome the blood–brain barrier:
- Intrathecal/intraventricular administration: Chemotherapy is injected directly into the cerebrospinal fluid, either through a lumbar puncture or a surgically implanted catheter.
- Intracerebral implants: A neurosurgeon creates a cavity within a tumor to allow the placement of dime-sized chemotherapy wafers, such as Gliadel wafers. Several of these wafers can be placed at the time of surgery and will release the chemotherapy agent carmustine slowly over time. This provides a much higher concentration of chemotherapy in the brain than can be obtained with intravenous administration, and it causes fewer systemic side effects. However, it is an option only for patients with surgically resectable tumours; it cannot be used to treat DIPG.[13]
- Osmotic blood–brain barrier disruption (BBBD): The cells of the blood–brain barrier are shrunk by a concentrated sugar solution (mannitol). This opens the barrier and allows 10 to 100 times more chemotherapy to enter the brain. A catheter is placed into a large artery (usually the one in the groin called the femoral artery) and threaded up to the carotid or vertebral artery. The hypertonic mannitol is injected, followed by a chemotherapeutic agent. Patients spend a few days in the hospital for each administration. This has been attempted with DIPG tumours.[14]
- Convection-enhanced delivery: Chemotherapy is delivered to the tumour by a surgically implanted catheter under a pressure gradient to achieve more distribution than with diffusion alone. Limited experiments have been conducted with brain tumors, including one with a DIPG.[15]
- Drug carriers: Carriers such as "Trojan horse" molecules, liposomes, and nanoparticles might theoretically allow a therapeutic drug to enter the brain. Such tactics are mostly in the investigatory stages and are not yet clinically relevant to brain tumour treatment.[12]
Development of Research Databases and Registries:
Starting in 2011 at the DIPG Symposium, under the cooperation of the DIPG Collaborative www.dipgcollaborative.org, The Cure Starts Now Foundation www.thecurestartsnow.org and 114 hospitals from 15 countries around the world, the International DIPG/DMG Registry was established creating the first linked platform of data collection for this disease. As of March, 2020, over 1100 patients[16][4] and families have contributed to this database making it the largest genomic, tissue, demographic and radiological database for DIPG and DMG in the world.
In 2019, The Cure Starts Now Foundation and the DIPG Collaborative partnered with the International DIPG Registry to develop www.dipg.org as a source of all current DIPG and DMG data, developed from current studies and expert articles by leading professionals.[17]
Prominent patients
- Karen Armstrong (1959–1962), daughter of American astronaut Neil Armstrong and his first wife, Janet Elizabeth Shearon.[18]
- Elena Desserich (2000–2007), daughter of Brooke and Keith Desserich. After her passing her parents founded The Cure Starts Now Foundation,[19] the first international DIPG/DMG charity that today has funded over $12 million in research at 114 hospitals. Her story was also chronicled in Notes Left Behind and became a New York Times bestselling book on November 12, 2009.[20]
- Gabriella Miller (2003–2013), American childhood cancer awareness advocate who raised thousands of dollars for childhood cancer charities and founded Smashing Walnuts Foundation.[21][22] The Gabriella Miller Kids First Research Act, signed into US law in 2014, was named after her.
- Lauren Hill (1995–2015), American freshman basketball player of Mount St. Joseph University, Cincinnati. To fulfil her wish of playing basketball for the college team for one game, the 2014 Hiram vs. Mount St. Joseph women's basketball game was scheduled 13 days earlier than the initially planned date and carried a brain cancer awareness message. Her efforts resulted in over $2.2 million raised for DIPG research through The Cure Starts Now Foundation.[23]
In popular culture
Notes Left Behind, a non-fictional book published in 2009, is about a girl named Elena Desserich who left hundreds of notes to her family before she died of DIPG at age 6.
| Wikimedia Commons has media related to Diffuse intrinsic pontine glioma. |
References
- ↑ American Brain Tumour Foundation http://www.abta.org/siteFiles/SitePages/1DA98D1B9B8D924603E99AA4C241B3A5.pdf Archived 2012-04-16 at the Wayback Machine
- ↑ "Diffuse Intrinsic Pontine Glioma (DIPG)". Archived from the original on 13 April 2015. Retrieved 20 December 2018.
- ↑ "Diffuse Midline Gliomas". National Cancer Institute. n.d. Archived from the original on 8 October 2019. Retrieved 24 November 2019.
- 1 2 "DIPG Statistics".
- ↑ St Jude Children's Research Hospital http://www.stjude.org/stjude/v/index.jsp?vgnextoid=b86c061585f70110VgnVCM1000001e0215acRCRD&vgnextchannel=bc4fbfe82e118010VgnVCM1000000e2015acRCRD
- ↑ childhood brain tumor http://www.childhoodbraintumor.org/medical-information/brain-tumor-types-and-imaging/item/81-brain-stem-gliomas-in-childhood.
- ↑ Fisher PG, Breiter SN, Carson BS, Wharam MD, Williams JA, Weingart JD, Foer DR, Goldthwaite PT, Burger PC. A clinicopathologic reappraisal of brainstem tumour classification: identification of pilocytic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer 89:1569–1576, 2000.
- ↑ Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brain stem gliomas. J Clin Oncol 24:1266–1272, 2006.
- ↑ Treatment of newly diagnosed diffuse brain stem gliomas in children – David N. Korones. http://www.expert-reviews.com/doi/abs/10.1586/14737140.7.5.663
- 1 2 3 "Recurrence/Relapse – DIPG Registry". Archived from the original on 9 April 2015. Retrieved 20 December 2018.
- ↑ Just One More Day
- 1 2 Getting into the Brain: Approaches to enhance brain drug delivery CNS Drugs 2009;23(1):35–58.
- ↑ "Archived copy". Archived from the original on 2010-05-05. Retrieved 2015-04-13.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Osmotic blood-brain barrier – disruption chemotherapy for diffuse pontine gliomas|J Neurooncol. 2006 May;77(3):279-84. Epub 2005 Nov 29
- ↑ Real-time image-guided direct convection perfusion of intrinsic brainstem lesions.|J Neurosurg. 2007 Jul;107(1):190-7.
- ↑ "International DIPG Registry Update: August 2018 | DIPG Registry".
- ↑ "DIPG: The Diffuse Intrinsic Pontine Glioma Resource Network".
- ↑ Hansen, James R. (2005). First Man: The Life of Neil A. Armstrong. New York: Simon & Schuster. pp. 161–164. ISBN 978-0-7432-5631-5. OCLC 937302502.
- ↑ https://www.thecurestartsnow.org/
- ↑ http://www.notesleftbehind.com/
- ↑ Gibson, Caitlin (14 November 2013). "Federal pediatric medical research act named for Gabriella Miller". The Washington Post. Retrieved 3 February 2021.
- ↑ "Our Story". Smashing Walnuts Foundation. Retrieved 3 February 2021.
- ↑ "Cure Starts Now reaches $2.2M goal in honor of Lauren Hill". Local 12. Retrieved 2021-05-03.
.png.webp)