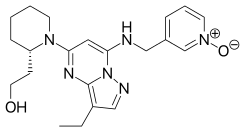
Dinaciclib
 | |
| Clinical data | |
|---|---|
| Other names | SCH-727965 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.246.885 |
| Chemical and physical data | |
| Formula | C21H28N6O2 |
| Molar mass | 396.495 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Dinaciclib (SCH-727965) is an experimental drug that inhibits cyclin-dependent kinases (CDKs).[1] It is being evaluated in clinical trials for various cancer indications.[2]
Dinaciclib is being developed by Merck & Co. It was granted orphan drug status by the FDA in 2011.[3]
Mechanisms of action
Anti-tumoral action
- In melanoma
- The anti-melanoma activity of dinaciclib is dependent on p53 signaling.[6]
- In chronic lymphocytic leukemia (CLL)
- In pancreatic cancer
- Dinaciclib inhibits pancreatic cancer growth and progression in murine xenograft models.[8]
- In osteosarcoma
Role in developing neurons
In primary cultured neurons, dinaciclib regulates neurogenesis, where it reduces expression of upper layer marker Satb2, and induces CTIP2, expressed in neurons of deeper layers.[11]
Clinical trials
References
- ↑ Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, Seghezzi W, Paruch K, Dwyer MP, Doll R, Nomeir A, Windsor W, Fischmann T, Wang Y, Oft M, Chen T, Kirschmeier P, Lees EM (Aug 2010). "Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor". Molecular Cancer Therapeutics. 9 (8): 2344–53. doi:10.1158/1535-7163.MCT-10-0324. PMID 20663931.
- ↑ Bose P, Simmons GL, Grant S (Jun 2013). "Cyclin-dependent kinase inhibitor therapy for hematologic malignancies". Expert Opinion on Investigational Drugs. 22 (6): 723–38. doi:10.1517/13543784.2013.789859. PMC 4039040. PMID 23647051.
- ↑ "Dinaciclib". AdisInsight. Retrieved 31 January 2017.
- ↑ Martin MP, Olesen SH, Georg GI, Schönbrunn E (Nov 2013). "Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains". ACS Chemical Biology. 8 (11): 2360–5. doi:10.1021/cb4003283. PMC 3846258. PMID 24007471.
- ↑ Nguyen TK, Grant S (Mar 2014). "Dinaciclib (SCH727965) inhibits the unfolded protein response through a CDK1- and 5-dependent mechanism". Molecular Cancer Therapeutics. 13 (3): 662–74. doi:10.1158/1535-7163.MCT-13-0714. PMC 3970263. PMID 24362465.
- ↑ Desai BM, Villanueva J, Nguyen TT, Lioni M, Xiao M, Kong J, Krepler C, Vultur A, Flaherty KT, Nathanson KL, Smalley KS, Herlyn M (2013). "The anti-melanoma activity of dinaciclib, a cyclin-dependent kinase inhibitor, is dependent on p53 signaling". PLOS ONE. 8 (3): e59588. Bibcode:2013PLoSO...859588D. doi:10.1371/journal.pone.0059588. PMC 3601112. PMID 23527225.
- ↑ Johnson AJ, Yeh YY, Smith LL, Wagner AJ, Hessler J, Gupta S, Flynn J, Jones J, Zhang X, Bannerji R, Grever MR, Byrd JC (Dec 2012). "The novel cyclin-dependent kinase inhibitor dinaciclib (SCH727965) promotes apoptosis and abrogates microenvironmental cytokine protection in chronic lymphocytic leukemia cells". Leukemia. 26 (12): 2554–7. doi:10.1038/leu.2012.144. PMC 3645353. PMID 22791353.
- ↑ Feldmann G, Mishra A, Bisht S, Karikari C, Garrido-Laguna I, Rasheed Z, Ottenhof NA, Dadon T, Alvarez H, Fendrich V, Rajeshkumar NV, Matsui W, Brossart P, Hidalgo M, Bannerji R, Maitra A, Nelkin BD (Oct 2011). "Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models". Cancer Biology & Therapy. 12 (7): 598–609. doi:10.4161/cbt.12.7.16475. PMC 3218385. PMID 21768779.
- ↑ Fu W, Ma L, Chu B, Wang X, Bui MM, Gemmer J, Altiok S, Pledger WJ (Jun 2011). "The cyclin-dependent kinase inhibitor SCH 727965 (dinacliclib) induces the apoptosis of osteosarcoma cells". Molecular Cancer Therapeutics. 10 (6): 1018–27. doi:10.1158/1535-7163.MCT-11-0167. PMC 4727401. PMID 21490307.
- ↑ Fu W, Sharma SS, Ma L, Chu B, Bui MM, Reed D, Pledger WJ (2013). "Apoptosis of osteosarcoma cultures by the combination of the cyclin-dependent kinase inhibitor SCH727965 and a heat shock protein 90 inhibitor". Cell Death & Disease. 4 (3): e566. doi:10.1038/cddis.2013.101. PMC 3613821. PMID 23538447.
- ↑ Ambrozkiewicz, Mateusz Cyryl; Bessa, Paraskevi; Salazar-Lázaro, Andrea; Salina, Valentina; Tarabykin, Victor (11 01, 2017). "Satb2Cre/+ mouse as a tool to investigate cell fate determination in the developing neocortex". Journal of Neuroscience Methods. 291: 113–121. doi:10.1016/j.jneumeth.2017.07.023. ISSN 1872-678X. PMID 28782628. S2CID 140208929.
{{cite journal}}: Check date values in:|date=(help) - ↑ Mita MM, Joy AA, Mita A, Sankhala K, Jou YM, Zhang D, Statkevich P, Zhu Y, Yao SL, Small K, Bannerji R, Shapiro CL (Jun 2014). "Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer". Clinical Breast Cancer. 14 (3): 169–76. doi:10.1016/j.clbc.2013.10.016. PMID 24393852.
- ↑ Stephenson JJ, Nemunaitis J, Joy AA, Martin JC, Jou YM, Zhang D, Statkevich P, Yao SL, Zhu Y, Zhou H, Small K, Bannerji R, Edelman MJ (Feb 2014). "Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer". Lung Cancer. 83 (2): 219–23. doi:10.1016/j.lungcan.2013.11.020. PMID 24388167.
- ↑ https://clinicaltrials.gov/ct2/show/study/NCT01096342
- ↑ https://clinicaltrials.gov/ct2/show/NCT00937937
- ↑ https://clinicaltrials.gov/ct2/show/study/NCT01580228
External links
- dinaciclib at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.