ELQ-300
 | |
| Names | |
|---|---|
| Preferred IUPAC name
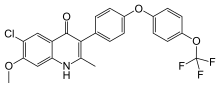
6-Chloro-7-methoxy-2-methyl-3-{4-[4-(trifluoromethoxy)phenoxy]phenyl}quinolin-4(1H)-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C24H17ClF3NO4 |
| Molar mass | 475.85 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
ELQ-300 is an experimental antimalarial medication. It is the first entry in a new class of antimalarials known as 4-quinolone-3-diarylethers.[1]
ELQ-300 acts as an inhibitor of the mitochondrial cytochrome bc1 complex (complex III in the electron transport chain) - A mechanism shared with some of the most potent fungicides known, the strobilurins.[1] In preclinical studies with mice, ELQ-300 was found to be highly active against Plasmodium falciparum and Plasmodium vivax at all life cycle stages that play a role in the transmission of malaria, and to have good oral bioavailability.[1]
References
- 1 2 3 Nilsen A; et al. (2013). "Quinolone-3-diarylethers: a new class of antimalarial drug". Science Translational Medicine. 5 (177): 177ra37. doi:10.1126/scitranslmed.3005029. ISSN 1946-6234. PMC 4227885. PMID 23515079.
Further reading
- "NIH-Supported Researchers Identify New Class of Malaria Compounds" (Press release). U.S. National Institutes of Health. March 20, 2013.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.