Enarodustat
 | |
| Clinical data | |
|---|---|
| Trade names | Enaroy |
| Other names | JTZ-951 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
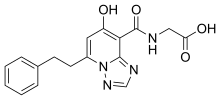
| Formula | C17H16N4O4 |
| Molar mass | 340.339 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Enarodustat (development code JTZ-951; brand name Enaroy) is a drug used for the treatment of anemia, especially when associated with chronic kidney disease (CKD). Enarodustat functions as a inhibitor of hypoxia inducible factor-proly hydroxylase (HIF-PH).[1]
The drug was approved in September 2020 in Japan for anemia associated with CKD[2] and is currently in clinical development in the United States and South Korea.[3] The drug is being developed by Japan Tobacco and JW Pharmaceutical.[4]
References
- ↑ Hirota K (April 2021). "HIF-α Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review". Biomedicines. 9 (5): 468. doi:10.3390/biomedicines9050468. PMC 8146675. PMID 33923349.
- ↑ "JT Receives Manufacturing and Marketing Approval of ENAROY Tablets 2mg・4mg for the Treatment of Anemia Associated with Chronic Kidney Disease in Japan" (PDF) (Press release). September 25, 2020.
- ↑ Markham A (January 2021). "Enarodustat: First Approval". Drugs. 81 (1): 169–174. doi:10.1007/s40265-020-01444-3. PMID 33320297. S2CID 229163684.
- ↑ "Enarodustat - Japan Tobacco". Adis Insight.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.