Enramycin
 | |
| Clinical data | |
|---|---|
| ATCvet code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C107H138Cl2N26O31 |
| Molar mass | 2355.33 g·mol−1 |
Enramycin (also knownb as enduracidin) is a polypeptide antibiotic produced by Streptomyces fungicidus. Enramycin is widely used as a feed additive for pigs and chickens to prevent necrotic enteritis induced by Gram-positive gut pathogens.[1]
Mechanism of action
Enramycin acts as an inhibitor of the enzyme MurG, which is essential for cell wall biosynthesis in Gram-positive bacteria. MurG catalyzes the transglycosylation reaction in the last step of peptidoglycan biosynthesis. Inhibiting this step greatly compromises cell wall integrity leading to cell lysis.[2]
Spectrum of susceptibility
Enramycin has been found to be very effective against Gram-positive gut pathogens, most notably, Clostridium perfringens; a leading cause of necrotic enteritis. The following represents MIC data for a couple of veterinary pathogens.[3]
- Clostridium perfringens: 0.05 μg/ml – 1.6 μg/ml
- Staphylococcus aureus: 0.013 μg/ml – 0.413 μg/ml
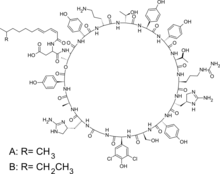
Composition
Standard grade enramycin is composed of two main components called enramycin A and enramycin B. These two components are routinely used as analytical reference standards; however, their activity as individual compounds does not appear to be widely studied or characterized.[4]
References
- ↑ Schering-Plough. "Enramycin." Darou Gostar Group. N.p., n.d. Web. 13 Mar. 2014.
- ↑ Fang X, Tiyanont K, Zhang Y, Wanner J, Boger D, Walker S (January 2006). "The mechanism of action of ramoplanin and enduracidin". Molecular BioSystems. 2 (1): 69–76. doi:10.1039/b515328j. PMID 16880924.
- ↑ "Enramycin Susceptibility and Resistance Data" (PDF).
- ↑ Inoue K, Hattori Y, Hino T, Oka H (April 2010). "An approach to on-line electrospray mass spectrometric detection of polypeptide antibiotics of enramycin for high-speed counter-current chromatographic separation". Journal of Pharmaceutical and Biomedical Analysis. 51 (5): 1154–60. doi:10.1016/j.jpba.2009.11.010. PMID 20004073.