Etabonate
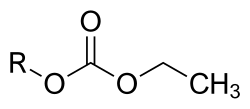
Etabonate or ethyl carbonate is the chemical group with formula –CO
3–C
2H
5, or H
3C–CH
2–O–C(=O)–O–. The names are also used for esters R–OCO
2C
2H
5, for the anion [C
2H
5OCO−
2], and for salts of the latter.
Pharmaceutical aspects
"Etabonate" is an international nonproprietary name (INN) and United States Adopted Name (USAN) for pharmaceutical substances and is the name almost universally used in pharmacology.[1] some important drugs featuring that group are
- Loteprednol etabonate, a corticosteroid
- Remogliflozin etabonate, an anti-diabetic drug
- Sergliflozin etabonate, another anti-diabetic drug
Chemical aspects
"Ethyl carbonate"is the name commonly used in chemistry. Important non-medical esters with that group are
- Diethyl carbonate, (H
3C–CH
2–O–)
2C=O - methyl ethyl carbonate, (H
3C–OC(=O)O–C
2H
5
Alkali salts of that anion, such as sodium ethyl carbonate, are known and fairly stable at ambient conditions. They have been proposed as an economical and environmentally friendly intermediate for the synthesis of organic compounds from carbon dioxide.[2] They can be obtained by reaction of carbon dioxide with alkali ethoxides:[2][3]
- [C
2H
5O−
] [M+
] + CO
2 → [C
2H
5OCO−
2] [M+
]
where M could be sodium or potassium. The ethoxide could be produced on a laboratory scale by reaction of ethanol with alkali metal,
- C
2H
5OH + M → [C
2H
5O−
] [M+
] + ½H
2
or, more economically and safely, by reaction with alkali hydroxide
- C
2H
5OH + MOH → [C
2H
5O−
] [M+
] + H
2O
at about 80 °C, using calcium oxide (quicklime) as a dehydrating agent to drive the reaction forward.[2]
Sodium ethyl carbonate is reported to be a white crystalline solid, nearly insoluble in many organic solvents (including diethyl ether, ethanol, acetone, and benzene), that decomposes without melting at 380-400 °C.[2][4]
In contrast, the hypothetical conjugate ethylcarbonic acid HOC(=O)O–C
2H
5 is not known and (like carbonic acid) may be stable only at very low temperatures.
History
Ethyl carbonate esters were described in 1837 by Jean-Baptiste André Dumas (1800-1884) and his assistant Eugène-Melchior Peligot (1811-1890), during their investigation of the nature of sugars (which had been incorrectly conjectured to be the same compounds).[5][6][7][8][9][10]
See also
- Retrometabolic drug design
- Methyl carbonate
- Dimethyl carbonate
References
- ↑ International Nonproprietary Names (INN) for pharmaceutical substances: Names for radicals, groups & others (PDF), World Health Organization, 2012, p. 28
- 1 2 3 4 Kh. A. Suerbaev, N. Zh. Kudaibergenov, N. R. Yesenzhanova, M. K. Kozhakhmet, and A. Gaini (2017): "Alkaline salts of alkyl carbonic acids as carboxylation reagents of phenols and naphtols". Известия Национальной академии наук Республики Казахстан (News of the Academy of Sciences of the Republic of Kazakhstan), Series Chemistry and Technology, volume 3, issue 423, pages 79-93. Quote: "[...] sodium and potassium ethyl carbonates are effective carboxylating reagents of hydroxyarenes (phenols and naphthols) [such as] hydroxybenzoic and hydroxynaphtoic acids." ISSN 2224-5286.
- ↑ Kh. A. Suerbaev, O. E. Mikhnenko, G. B. Akhmetova, K. M. Shalmagambetov, and E. G. Chepaikin (2005): "Phenol carboxylation with alkali metal salts of ethyl carbonic acid". Petroleum Chemistry, volume 45,issue 1, pages 41-43. Quote: "[...] phenol carboxylation with sodium ethyl carbonate under optimal conditions (P-Ar = 1 MPa, T = 200 °C, tau = 6 h) yielded o-hydroxybenzoic acid (69.9%) and p-hydroxybenzoic acid (17.5%). [...] preparation of p-hydroxybenzoic acid by phenol carboxylation with potassium ethyl carbonate (P-CO2 = 2.5 MPa, T= 210 °C, and tau = 7 h) in a 71% yield was developed".
- ↑ S. J. Han and J. H. Wee (2016): "Carbon dioxide fixation via synthesis of sodium ethyl carbonate in NaOH-dissolved ethanol", Industrial Engineering & Chemical Research, volume 55, pages 12111-12118. doi:10.1021/acs.iecr.6b03250
- ↑ Jaime Wisniak (2009): "Eugène Melchior Peligot". Educación Química, volume 20, issue 1, pages 61-69. doi:10.1016/S0187-893X(18)30008-9
- ↑ J.-B. Dumas and E. Peligot (1837): "Note sur le carbométhylate de baryte". Comptes Rendus, volume 2, pages 433-434.
- ↑ J.-B. Dumas and E. Peligot (1837): "Sur le carbovinate de potasse". Comptes Rendus, volume 4 pages 563-565.
- ↑ J.-B Dumas, L.J. Thenard, J.L. Gay-Lussac, and J.B. Biot (1838): "Rapport sur un mémoire de M. Peligot, intitulé: Recherches sur la nature et les propriétés chimiques des sucres". Comptes Rendus, volume 7 pages 106-113.
- ↑ M.E. Jungfleish (1891): "Notice sur la vie et les travaux - Eugène Melchior Peligot". Bulletin de la Société Chimique, volume 5, pages xxi-xlvii, 1890
- ↑ M.E. Jungfleish (1891): "Notice sur la vie et les travaux - Eugène Melchior Peligot". Annales du Conservatoire des arts et métiers, volume 2, pages 85-102.