Eteplirsen
 | |
| Names | |
|---|---|
| Trade names | Exondys 51 |
| Other names | AVI-4658 |
IUPAC name
| |
| Clinical data | |
| Drug class | Antisense oligonucleotide[1] |
| Main uses | Duchenne muscular dystrophy (DMD)[2] |
| Side effects | Problems with balance, vomiting, allergic reactions[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intravenous infusion |
| Typical dose | 30 mg/kg q wk[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C364H569N177O122P30 |
| Molar mass | 10305.886 g·mol−1 |
SMILES
| |
InChI
| |
Eteplirsen, sold under the brand name Exondys 51, is a medication used to treat Duchenne muscular dystrophy (DMD) with specific mutations.[2] These specific mutations are present in about 14% of DMD cases.[2] Meaningful benefit in this group; however, is unclear.[2] It is given by injection into a vein.[1]
Common side effects include problems with balance, vomiting, and contact dermatitis.[1][2] Other health problems may include allergic reactions.[1] It is a form of antisense therapy which results in exon 51 skipping.[1]
Eteplirsen was approved for medical use in the United States in 2016.[2] Approval was so controversial that two FDA review panel members resigned in protest.[3] It use was not approved in Europe due to insufficient evidence.[4] In the United States the cost for a child weighting 20 kg is about 524,000 USD per year.[5]
Medical uses
Eteplirsen is used to treat Duchenne muscular dystrophy (DMD) with specific mutations.[2] It targets a specific mutations found in about 14% of people with DMD.[6][7]
In this group it leads to about a quarter of a percent (0.25%) increase dystrophin; though it is unclear if such an increase results in any benefit for the person in question.[3] The overall benefits versus risks were found to be unclear by the EMA.[4]
Dosage
It is given at a dose of 30 mg/kg once per week.[1]
Side effects
The following adverse events were observed in at least 10% of people who received eteplirsen in trials: vomiting, contusion, excoriation, arthralgia, rash, catheter site pain, and upper respiratory tract infection.[8]
Mechanism of action
Duchenne muscular dystrophy is caused when a mutation in the DMD gene changes the DMD RNA so that it no longer codes for functional dystrophin protein, usually due to a mutation that alters the reading frame of the RNA downstream of the mutation. If an exon with an appropriate number of bases lies near the mutation, by removing that exon the downstream reading frame can be corrected and production of partially functional dystrophin can be restored. This is the general strategy used for designing exon-skipping oligos for DMD; as there are 79 exons in the longest splice form of the dystrophin transcript, many different oligos are needed to address the range of mutations present in the population of people with DMD.
Eteplirsen is a morpholino antisense oligomer which triggers excision of exon 51 during pre-mRNA splicing of the dystrophin RNA transcript. Skipping exon 51 changes the downstream reading frame of dystrophin;[9] giving eteplirsen to a healthy person would result in production of dystrophin mRNA which would not code for functional dystrophin protein but, for DMD patients with particular frameshifting mutations, giving eteplirsen can restore the reading frame of the dystrophin mRNA and result in production of functional (although modified by having an internal deletion consisting of both the patient's original defect, as well as the therapeutically skipped exon) dystrophin.[10] Eteplirsen is given by intravenous infusion for systemic treatment of DMD.
Exon skipping is induced by eteplirsen, a charge-neutral, phosphorodiamidate morpholino oligomer (PMO) that selectively binds to exon 51 of dystrophin pre-mRNA, restoring the phase of the reading frame and enabling production of functional, but internally edited, dystrophin.[11] The uncharged nature of the PMO helps make it resistant to biological degradation.[12] This modified dystrophin protein produced by eteplirsen may cause a less severe form of dystrophinopathy, much like Becker muscular dystrophy. Eteplirsen's proposed mechanism of action is to bind to dystrophin pre-mRNA, alter the exon splicing of the RNA so that more dystrophin is made. By increasing the quantity of an abnormal, but potentially functional, dystrophin protein, the objective is to slow or prevent the progression of DMD.[11][13]
Oligo and target
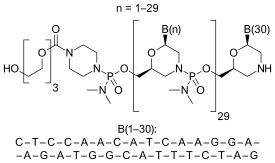
Eteplirsen is a morpholino phosphorodiamidate antisense oligomer.
CTCCAACATCAAGGAAGATGGCATTTCTAG (sequence source: US FDA ETEPLIRSEN BRIEFING DOCUMENT NDA 206488[11]),
30-mer,
20% G,
43% CG,
Predicted Tm: 88.9 °C at 10 μM oligo.
Oligo complement CTAGAAATGCCATCTTCCTTGATGTTGGAG
DMD-001 Exon 51, ENST00000357033.8 in Ensembl.org, RNA target site marked. Given that the target site is within an exon, this is likely blocking binding of an exonic splice enhancer protein and so altering splicing by interfering with splice regulation. CTCCTACTCAGACTGTTACTCTGGTGACACAACCTGTGGTTACTAAGGAAACTGCCATCT CCAAA[CTAGAAATGCCATCTTCCTTGATGTTGGAG]GTACCTGCTCTGGCAGATTTCAACC GGGCTTGGACAGAACTTACCGACTGGCTTTCTCTGCTTGATCAAGTTATAAAATCACAGA GGGTGATGGTGGGTGACCTTGAGGATATCAACGAGATGATCATCAAGCAGAAG
Pharmacokinetics
Following single or multiple intravenous infusions, the majority of drug elimination occurred within 24 hours of intravenous administration. Elimination half-life of eteplirsen was 3 to 4 hours.[8]
History
New Drug Applications (NDA) for eteplirsen and a similar drug drisapersen were filed with the US Food and Drug Administration (FDA) in August 2015.[14] The Prescription Drug User Fee Act (PDUFA) goal dates for these were December 27, 2015 for drisapersen and February 26, 2016 for eteplirsen. Following FDA rejection of drisapersen, the agency announced a three-month time extension for its review of eteplirsen. The FDA panel decision was controversial because the FDA staff and the panel used a stricter standard of evidence than Sarepta and patient groups used. The FDA panel said that it was required by law to apply the standard of "substantial evidence" of effectiveness. This required randomized, controlled trials showing effectiveness of a meaningful clinical outcome, such as the ability to function in daily life. Sarepta and patient groups wanted to use the standard of historical controls, personal testimonies, and the presence of altered dystrophin in the body. On April 25, 2016, the Advisory Committee Panel voted against approval;.[15] However, in June 2016, FDA requested for additional data from Sarepta to confirm findings of dystrophin production by eteplirsen. Janet Woodcock, director of the FDA's Center for Drug Evaluation and Research, overruled the panel, and FDA Commissioner Robert Califf deferred to her decision. Eterplirsen received accelerated approval on September 19, 2016.[16]
The European Medicines Agency reviewed the molecule in 2018, and refused to approve it.[4][17]
Following the approval of eteplirsen, two other drugs of a similar kind, golodirsen and viltolarsen received provisional approval from the FDA for the treatment of people with a confirmed mutation of the dystrophin gene that is amenable to exon 53 skipping[18][19] as well as casimersen for exon 45 skipping.[20]
Society and culture
Economics
The US list price of eteplirsen is US$300,000 per year of treatment. The Institute for Clinical and Economic Review has found the drug not cost effective at the list price when the cost of one Quality-adjusted life year (QALY) was equal to US$150,000.[21]
References
- 1 2 3 4 5 6 7 8 "DailyMed - EXONDYS 51- eteplirsen injection". dailymed.nlm.nih.gov. Archived from the original on 3 August 2021. Retrieved 16 December 2021.
- 1 2 3 4 5 6 7 "Eteplirsen Monograph for Professionals". Drugs.com. Retrieved 16 December 2021.
- 1 2 "Railroading at the FDA". Nature Biotechnology. 34 (11): 1078. November 2016. doi:10.1038/nbt.3733. PMID 27824847.
- 1 2 3 "Exondys EPAR". European Medicines Agency (EMA). Archived from the original on 26 November 2020. Retrieved 13 August 2020.
- ↑ "Exondys 51 Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 7 May 2021. Retrieved 16 December 2021.
- ↑ Scoto M, Finkel R, Mercuri E, Muntoni F (August 2018). "Genetic therapies for inherited neuromuscular disorders". The Lancet. Child & Adolescent Health. 2 (8): 600–609. doi:10.1016/S2352-4642(18)30140-8. PMID 30119719. Archived from the original on 2021-08-17. Retrieved 2021-09-06.
- ↑ Lim KR, Maruyama R, Yokota T (2017). "Eteplirsen in the treatment of Duchenne muscular dystrophy". Drug Design, Development and Therapy. 11: 533–545. doi:10.2147/DDDT.S97635. PMC 5338848. PMID 28280301.
Eteplirsen is applicable for approximately 14% of patients with DMDmutations
- 1 2 "Eteplirsen - prescribing information" (PDF). FDA. September 2016. Archived (PDF) from the original on 2021-06-27. Retrieved 2021-09-06.
- ↑ Anthony K, Feng L, Arechavala-Gomeza V, Guglieri M, Straub V, Bushby K, et al. (October 2012). "Exon skipping quantification by quantitative reverse-transcription polymerase chain reaction in Duchenne muscular dystrophy patients treated with the antisense oligomer eteplirsen". Human Gene Therapy Methods. 23 (5): 336–45. doi:10.1089/hgtb.2012.117. PMID 23075107.
- ↑ Moulton HM, Moulton JD (December 2010). "Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1798 (12): 2296–303. doi:10.1016/j.bbamem.2010.02.012. PMID 20170628.
- 1 2 3 "Eteplirsen" (PDF). FDA Briefing Document. 25 April 2016. Archived (PDF) from the original on 15 December 2018. Retrieved 6 September 2021.
- ↑ Kole R, Leppert BJ (July 2012). "Targeting mRNA splicing as a potential treatment for Duchenne muscular dystrophy". Discovery Medicine. 14 (74): 59–69. PMID 22846203. Archived from the original on 2020-07-26. Retrieved 2021-09-06.
- ↑ Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, et al. (November 2013). "Eteplirsen for the treatment of Duchenne muscular dystrophy". Annals of Neurology. 74 (5): 637–47. doi:10.1002/ana.23982. PMID 23907995. S2CID 24359589. Archived from the original on 2021-10-31. Retrieved 2021-09-06.
- ↑ "FDA Accepts Sarepta's NDA for Eteplirsen". Rare Disease Report. Archived from the original on 2015-08-28. Retrieved 2015-08-28.
- ↑ Pollack, Andrew (2016-04-25). "Advisers to F.D.A. Vote Against Duchenne Muscular Dystrophy Drug". The New York Times. Archived from the original on 2020-11-08. Retrieved 2021-09-06.
- ↑ "Column: To appease a patient lobby, did the FDA approve a $300,000 drug that doesn't work? Michael Hiltzik, Los Angeles Times, October 28, 2016". Archived from the original on October 19, 2021. Retrieved September 6, 2021.
- ↑ "Going Its Own Way, European Regulators Reject Sarepta's Exondys 51 for DMD". BioSpace. Archived from the original on 2019-12-14. Retrieved 2019-12-14.
- ↑ Anwar S, Yokota T (August 2020). "Golodirsen for Duchenne muscular dystrophy". Drugs of today. 56 (8): 491-504. doi:10.1358/dot.2020.56.8.3159186. PMID 33025945.
- ↑ Dhillon S (Jul 2020). "Viltolarsen: first approval". Drugs. 80 (10): 1027-1031. doi:10.1007/s40265-020-01339-3. PMID 32519222.
- ↑ "Archive copy". Archived from the original on 2021-08-03. Retrieved 2021-09-06.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "ICER sees current DMD therapies as too pricey, but notes data limitations". BioPharma Dive. Archived from the original on 2019-12-14. Retrieved 2019-12-14.
External links
| External sites: |
|
|---|---|
| Identifiers: |