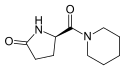
Fasoracetam
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(5R)-5-(Piperidine-1-carbonyl)pyrrolidin-2-one | |||
| Other names | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C10H16N2O2 | ||
| Molar mass | 196.250 g·mol−1 | ||
| Pharmacology | |||
| Oral | |||
| Legal status |
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Fasoracetam is a research chemical of the racetam family.[3] It is a putative nootropic that failed to show sufficient efficacy in clinical trials for vascular dementia. It is currently being studied for its potential use for attention deficit hyperactivity disorder.[2][4]
Fasoracetam appears to agonize all three groups of metabotropic glutamate receptors and has improved cognitive function in rodent studies.[5] It is orally bioavailable and is excreted mostly unchanged via the urine.[6]
Fasoracetam was discovered by scientists at the Japanese pharmaceutical company Nippon Shinyaku, which brought it through Phase 3 clinical trials for vascular dementia, and abandoned it due to lack of efficacy.[5][7]
Scientists at Children's Hospital of Philadelphia led by Hakon Hakonarson have studied fasoracetam's potential use in attention deficit hyperactivity disorder.[5] Hakonarson started a company called neuroFix Therapeutics to try to bring the drug to market for this use; neuroFix acquired Nippon Shinyaku's clinical data as part of its efforts.[7][8] neuroFix was acquired by Medgenics in 2015.[8] Medgenics changed its name to Aevi Genomic Medicine in 2016.[9] Clinical trials in adolescents with ADHD who also have mGluR mutations started in 2016.[8] While Fasoracetam may be effective in the treatment of ADHD in people with specific mGluR mutations, these represent around 10% of total ADHD cases, and Fasoracetam is likely ineffective in all other cases.[10][11] Studies showing improvements in cognitive function from Fasoracetam have exclusively been done on rodents.[12]
Legality
Australia
Fasoracetam is a schedule 4 substance in Australia under the Poisons Standard (February 2020).[13] A schedule 4 substance is classified as "Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by State or Territory legislation to prescribe and should be available from a pharmacist on prescription."[13]
See also
References
- ↑ FDA/NIH Substance registration system. Page accessed March 21, 2016
- 1 2 "Drug Profile Fasoracetam".
- ↑ "5-oxo-D-prolinepiperidinamide monohydrate - Compound Summary". Retrieved 21 July 2013.
- ↑ "Recommended INN List 40" (PDF). WHO Drug Information. 12 (2). 1998.
- 1 2 3 Connolly, J; Glessner, J; Kao, C; Elia, J; Hakonarson, H (2015). "ADHD & Pharmacotherapy: Past, Present and Future: A Review of the Changing Landscape of Drug Therapy for Attention Deficit Hyperactivity Disorder". Ther Innov Regul Sci. 49 (5): 632–642. doi:10.1177/2168479015599811. PMC 4564067. PMID 26366330.
- ↑ Malykh, AG; Sadaie, MR (12 February 2010). "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders". Drugs. 70 (3): 287–312. doi:10.2165/11319230-000000000-00000. PMID 20166767. S2CID 12176745.
- 1 2 Moskowitz, D. H. (2017). Finding the Genetic Cause and Therapy for ADHD, Autism and 22q. BookBaby (self published). ISBN 9781483590981.
- 1 2 3 Sharma, B. "Medgenics: NFC-1 Could Be A Key Future Revenue Driver".
- ↑ "Press Release: Medgenics, Inc. Announces Name Change to Aevi Genomic Medicine, Inc". Aevi via MarketWired. 16 December 2016.
- ↑ "Fasoracetam in adolescents with ADHD and glutamatergic gene network variants disrupting mGluR neurotransmitter signaling". ResearchGate. Retrieved 2020-10-16.
- ↑ Tardner, P. "Fasoracetam as a treatment for ADHD: A systematic review of available clinical data • International Journal of Environmental Science & Technology". International Journal of Environmental Science & Technology. Retrieved 2020-10-16.
{{cite web}}: CS1 maint: url-status (link) - ↑ Elia, Josephine; Ungal, Grace; Kao, Charlly; Ambrosini, Alexander; De Jesus-Rosario, Nilsa; Larsen, Lene; Chiavacci, Rosetta; Wang, Tiancheng; Kurian, Christine; Titchen, Kanani; Sykes, Brian (2018-01-16). "Fasoracetam in adolescents with ADHD and glutamatergic gene network variants disrupting mGluR neurotransmitter signaling". Nature Communications. 9 (1): 4. Bibcode:2018NatCo...9....4E. doi:10.1038/s41467-017-02244-2. ISSN 2041-1723. PMC 5770454. PMID 29339723.
- 1 2 Poisons Standard February 2020. comlaw.gov.au