Flock House virus
| Flock House virus | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Kitrinoviricota |
| Class: | Magsaviricetes |
| Order: | Nodamuvirales |
| Family: | Nodaviridae |
| Genus: | Alphanodavirus |
| Species: | Flock House virus |
Flock House virus (FHV) is in the alphanodavirus genus of the Nodaviridae family of viruses. Flock House virus was isolated from a grass grub (Costelytra Zealandica) at the Flock House research station in Bulls, New Zealand. FHV is an extensively studied virus and is considered a model system for the study of other non-enveloped RNA viruses owing to its small size and genetic tractability, particularly to study the role of the transiently exposed hydrophobic gamma peptide and the metastability of the viral capsid.[1][2] FHV can be engineered in insect cell culture allowing for the tailored production of native or mutant authentic virions or virus-like-particles. FHV is a platform for nanotechnology and nanomedicine, for example, for epitope display and vaccine development.[3] Viral entry into host cells occurs via receptor-mediated endocytosis.[4] Receptor binding initiates a sequence of events during which the virus exploits the host environment in order to deliver the viral cargo in to the host cytosol. Receptor binding prompts the meta-stability of the capsid–proteins, the coordinated rearrangements of which are crucial for subsequent steps in the infection pathway. In addition, the transient exposure of a covalently-independent hydrophobic γ-peptide is responsible for breaching cellular membranes and is thus essential for the viral entry of FHV into host cells.[5]
Genome
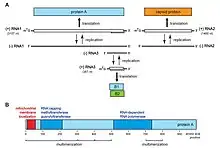
Flock House virus is a small, non-enveloped, icosahedral T=3 insect virus containing a bipartite positive sense ssRNA genome comprising two genes: RNA1 (3.1kb) an RNA2 (1.4kb). RNA1 encodes the RNA-dependent RNA polymerase and also contains a frame-shifted subgenomic RNA 3 (369 nts) that encodes protein B2, responsible for inhibition of RNAi pathways.[6] RNA2 encodes the capsid precursor, alpha, of which 180 copies form the viral capsid of FHV. Upon maturation, alpha undergoes an autocatalytic cleavage in its C-terminus to form beta, forming the main structural capsid component, and gamma, a short hydrophobic peptide required for endosome penetration that remains associated with the viral capsid. Virus-Like-Particles (VLPs) of FHV spontaneously form in S. frugiperda cell lines (e.g. Sf21) when RNA2 is expressed from a baculovirus vector and package cellular RNAs.[7][8]
Range
FHV was originally isolated from New Zealand grass grubs (Costelytra zealandica) in the former Flock House agricultural facility in Bulls, Ragnitikei, New Zealand.[9] Isolates were passaged in Drosophila cells in culture, which were subsequently shown to exhibit cell-death (cytopathic effect). FHV can also infect live flies.[10] FHV has been shown to infect medically important genera of insects: mosquitos, e.g. Anopheles gambiae; the tsetse fly; and the Chagas vector, Rhodnius prolixus Stal.[11][12] Infection of these organisms by FHV has been demonstrated to have similar characteristics in terms of viral titre, virus dissemination and mortality as has been shown for fruit fly infections.
Capsid structure
The structure and biophysical properties of authentic virions of FHV and of virus-like-particles (VLPs) have been extensively studied.
Other studies
FHV has provided a model system for the study of the emergence and evolution of defective-interfering RNAs (DI-RNAs).
References
- ↑ Odegard, A; Banerjee, M; Johnson, JE (2010). Flock house virus: a model system for understanding non-enveloped virus entry and membrane penetration. Current Topics in Microbiology and Immunology. Vol. 343. pp. 1–22. doi:10.1007/82_2010_35. ISBN 978-3-642-13331-2. PMID 20407886.
- ↑ Banerjee, M; Johnson, JE (February 2008). "Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry". Current Protein & Peptide Science. 9 (1): 16–27. doi:10.2174/138920308783565732. PMID 18336320.
- ↑ Destito, G; Schneemann, A; Manchester, M (2009). "Biomedical nanotechnology using virus-based nanoparticles". Current Topics in Microbiology and Immunology. 327: 95–122. doi:10.1007/978-3-540-69379-6_5. ISBN 978-3-540-69376-5. PMID 19198572.
- ↑ Odegard, AL; Kwan, MH; Walukiewicz, HE; Banerjee, M; Schneemann, A; Johnson, JE (September 2009). "Low endocytic pH and capsid protein autocleavage are critical components of Flock House virus cell entry". Journal of Virology. 83 (17): 8628–37. doi:10.1128/JVI.00873-09. PMC 2738175. PMID 19553341.
- ↑ Schneemann, A; Zhong, W; Gallagher, T. M; Rueckert, R. R (1992). "Maturation cleavage required for infectivity of a nodavirus". Journal of Virology. 66 (11): 6728–34. doi:10.1128/JVI.66.11.6728-6734.1992. PMC 240169. PMID 1404613.
- ↑ Chao, JA; Lee, JH; Chapados, BR; Debler, EW; Schneemann, A; Williamson, JR (November 2005). "Dual modes of RNA-silencing suppression by Flock House virus protein B2". Nature Structural & Molecular Biology. 12 (11): 952–7. doi:10.1038/nsmb1005. PMID 16228003. S2CID 37878393.
- ↑ Schneemann, A; Dasgupta, R; Johnson, JE; Rueckert, RR (May 1993). "Use of recombinant baculoviruses in synthesis of morphologically distinct viruslike particles of flock house virus, a nodavirus". Journal of Virology. 67 (5): 2756–63. doi:10.1128/JVI.67.5.2756-2763.1993. PMC 237599. PMID 8474173.
- ↑ Routh, A; Domitrovic, T; Johnson, JE (7 February 2012). "Host RNAs, including transposons, are encapsidated by a eukaryotic single-stranded RNA virus". Proceedings of the National Academy of Sciences of the United States of America. 109 (6): 1907–12. Bibcode:2012PNAS..109.1907R. doi:10.1073/pnas.1116168109. PMC 3277583. PMID 22308402.
- ↑ Scotti, PD; Dearing, S; Mossop, DW (1983). "Flock House virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae)". Archives of Virology. 75 (3): 181–9. doi:10.1007/BF01315272. PMID 6188442. S2CID 9853542.
- ↑ Goic, B; Vodovar, N; Mondotte, JA; Monot, C; Frangeul, L; Blanc, H; Gausson, V; Vera-Otarola, J; Cristofari, G; Saleh, MC (April 2013). "RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila" (PDF). Nature Immunology. 14 (4): 396–403. doi:10.1038/ni.2542. PMID 23435119. S2CID 6690552.
- ↑ Dasgupta, R; Cheng, LL; Bartholomay, LC; Christensen, BM (July 2003). "Flock house virus replicates and expresses green fluorescent protein in mosquitoes". The Journal of General Virology. 84 (Pt 7): 1789–97. doi:10.1099/vir.0.18938-0. PMID 12810873.
- ↑ Dasgupta, R; Free, HM; Zietlow, SL; Paskewitz, SM; Aksoy, S; Shi, L; Fuchs, J; Hu, C; Christensen, BM (January 2007). "Replication of flock house virus in three genera of medically important insects". Journal of Medical Entomology. 44 (1): 102–10. doi:10.1603/0022-2585(2007)44[102:rofhvi]2.0.co;2. PMID 17294927.