Fluorescence in situ hybridization
.gif)
Fluorescence in situ hybridization (FISH[1]) is a molecular cytogenetic technique that uses fluorescent probes that bind to only particular parts of a nucleic acid sequence with a high degree of sequence complementarity. It was developed by biomedical researchers in the early 1980s[2] to detect and localize the presence or absence of specific DNA sequences on chromosomes. Fluorescence microscopy can be used to find out where the fluorescent probe is bound to the chromosomes. FISH is often used for finding specific features in DNA for use in genetic counseling, medicine, and species identification.[3] FISH can also be used to detect and localize specific RNA targets (mRNA, lncRNA and miRNA) in cells, circulating tumor cells, and tissue samples. In this context, it can help define the spatial-temporal patterns of gene expression within cells and tissues.[1][4]
Probes – RNA and DNA

In biology, a probe is a single strand of DNA or RNA that is complementary to a nucleotide sequence of interest.RNA probes can be designed for any gene or any sequence within a gene for visualization of mRNA,[5][6][7] lncRNA[8][9][10] and miRNA in tissues and cells. FISH is used by examining the cellular reproduction cycle, specifically interphase of the nuclei for any chromosomal abnormalities.[11] FISH allows the analysis of a large series of archival cases much easier to identify the pinpointed chromosome by creating a probe with an artificial chromosomal foundation that will attract similar chromosomes.[11] The hybridization signals for each probe when a nucleic abnormality is detected.[11]
Each probe for the detection of mRNA and lncRNA is composed of ~20-50 oligonucleotide pairs, each pair covering a space of 40–50 bp. The specifics depend on the specific FISH technique used. For miRNA detection, the probes use proprietary chemistry for specific detection of miRNA and cover the entire miRNA sequence. The artificial chromosomes (BAC) can be grown, extracted, and labeled, in any lab containing a library, genomic libraries are often named after the institution in which they were developed. [12][13][14]
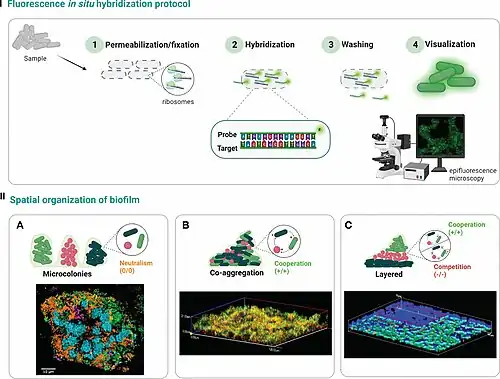
Preparation and hybridization process – RNA
The purpose of using RNA FISH is to detect target mRNA transcripts in cells, tissue sections, or even whole-mounts. The process is done in 3 main procedures: tissue preparation , hybridization, and washing .[15]
The tissue preparation starts by collecting the appropriate tissue sections to perform RNA FISH. First, cells, circulating tumor cells (CTCs), formalin-fixed paraffin-embedded (FFPE), or frozen tissue sections are fixed. Some commonly used fixatives are 4% formaldehyde or paraformaldehyde (PFA) in phosphate buffered saline (PBS).[15] FISH has also been successfully done on unfixed cells.[16] After fixation, samples are permeabilized to allow the penetration of hybridization reagents. The use of detergents at a 0.1% concentration is commonly used to enhance the tissue permeability such as Tween-20 or Triton X-100.[12]
It is critical for the hybridization process to have all optimal conditions to have a successful in situ result, including temperature, pH, salt concentration, and time of the hybridization reaction. After checking all the necessary conditions, hybridization steps can be started by first adding a target-specific probe, composed of 20 oligonucleotide pairs, hybridizes to the target RNA(s). Separate but compatible signal amplification systems enable the multiplex assay. Signal amplification is achieved via series of sequential hybridization steps.[17]
After the hybridization steps, washing steps are performed. These steps aim to remove nonspecific hybrids and get rid of unbound probe molecules from the samples to reduce any background signaling. The use of ethanol washes are typically used at this stage to reduce autofluorescence in tissues or cells.[18] At the end of the assay the tissue samples are visualized under a fluorescence microscope such as the confocal fluorescence microscope and the Keyence microscope.[12]
Preparation and hybridization process – DNA
.jpg.webp)
First, a probe is constructed, it must be large enough to hybridize specifically with its target but not so large as to impede the hybridization process. The probe is tagged directly with fluorophores, with targets for antibodies or with biotin. Tagging can be done in various ways, such as nick translation, or polymerase chain reaction using tagged nucleotides.Then, an interphase or metaphase chromosome preparation is produced, the chromosomes are firmly attached to a substrate , usually glass. Repetitive DNA sequences must be blocked by adding short fragments of DNA to the sample. The probe is then applied to the chromosome DNA and incubated for approximately 12 hours while hybridizing. Several wash steps remove all unhybridized or partially hybridized probes. The results are then visualized and quantified using a microscope that is capable of exciting the dye and recording images.If the fluorescent signal is weak, amplification of the signal may be necessary in order to exceed the detection threshold of the microscope. Fluorescent signal strength depends on many factors such as probe labeling efficiency, the type of probe, and the type of dye. Fluorescently tagged antibodies or streptavidin are bound to the dye molecule, these secondary components are selected so that they have a strong signal.[19][20][15][21]
Variations on probes and analysis
FISH is a very general technique, the differences between the various FISH techniques are usually due to variations in the sequence and labeling of the probes; and how they are used in combination. Probes are divided into two general categories- cellular and acellular. In fluorescent "in situ" hybridization refers to the cellular placement of the probe.Probe size is important because shorter probes hybridize less specifically than longer probes, so that long enough strands of DNA or RNA which are complementary to a given target sequence are often used to locate a target. The overlap defines the resolution of detectable features. For example, if the goal of an experiment is to detect the breakpoint of a translocation, then the overlap of the probes, defines the minimum window in which the breakpoint may be detected.[1][22][15]
The mixture of probe sequences determines the type of feature the probe can detect. Probes that hybridize along an entire chromosome are used to count the number of a certain chromosome, show translocations, or identify extra-chromosomal fragments of chromatin.[23] This is often called "whole-chromosome painting." If every possible probe is used, every chromosome, would be marked fluorescently, which would not be particularly useful for determining features of individual sequences. However, it is possible to create a mixture of smaller probes that are specific to a particular region (locus) of DNA; these mixtures are used to detect deletion mutations. When combined with a specific color, a locus-specific probe mixture is used to detect very specific translocations. Special locus-specific probe mixtures are often used to count chromosomes, by binding to the centromeric regions of chromosomes, which are distinctive enough to identify each chromosome .A variety of other techniques uses mixtures of differently colored probes. A range of colors in mixtures of fluorescent dyes can be detected, so each human chromosome can be identified by a characteristic color using whole-chromosome probe mixtures and a variety of ratios of colors. Similar to comparative genomic hybridization, the probe mixture for the secondary colors is created by mixing the correct ratio of two sets of differently colored probes for the same chromosome; this technique is sometimes called M-FISH.The same physics that make a variety of colors possible for M-FISH can be used for the detection of translocations. Some assays are designed so that the secondary color will be present or absent in cases of interest. An example is the detection of BCR/ABL translocations, where the secondary color indicates disease. This variation is often called double-fusion FISH color is observed, but only the primary colors are observed when the translocation occurs.[24][25][22][26]
Where the absence of the secondary color is pathological, is illustrated by an assay used to investigate translocations where only one of the breakpoints is known or constant; Locus-specific probes are made for one side of the breakpoint and the other intact chromosome. [27]
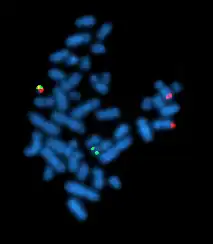 A metaphase cell positive for the bcr/abl rearrangement using FISH
A metaphase cell positive for the bcr/abl rearrangement using FISH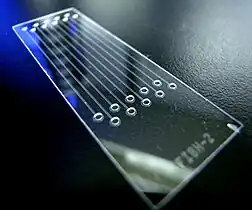 Microfluidic chip that lowered the cost-per-test of FISH
Microfluidic chip that lowered the cost-per-test of FISH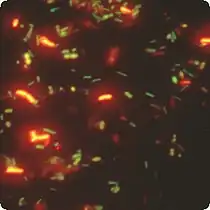 Dual label FISH image; Bifidobacteria Cy3, Total bacteria FITC.
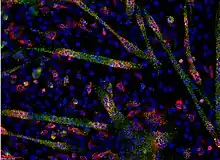
Dual label FISH image; Bifidobacteria Cy3, Total bacteria FITC.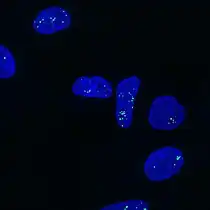 Paraspeckles visualized by single-molecule FISH against NEAT1
Paraspeckles visualized by single-molecule FISH against NEAT1
Single-molecule RNA FISH
Single-molecule RNA FISH, also known as Stellaris® RNA FISH[13] or smFISH,[28] is a method of detecting and quantifying mRNA and other long RNA molecules in a thin layer of tissue sample. Targets can be reliably imaged through the application of multiple short singly labeled oligonucleotide probes.[29] The binding of up to 48 fluorescent labeled oligos to a single molecule of mRNA provides sufficient fluorescence to accurately detect and localize each target mRNA in a wide-field fluorescent microscopy image. Probes not binding to the intended sequence do not achieve sufficient localized fluorescence to be distinguished from background.[30]
Single-molecule RNA FISH assays can be performed in simplex or multiplex, and can be used as a follow-up experiment to quantitative PCR, or imaged simultaneously with a fluorescent antibody assay. The technology has potential applications in cancer diagnosis,[31] neuroscience, gene expression analysis,[32] and companion diagnostics.
Fiber FISH
In an alternative technique to interphase or metaphase preparations, fiber FISH, interphase chromosomes are attached to a slide in such a way that they are stretched out in a straight line, rather than being tightly coiled, as in conventional FISH, or adopting a chromosome territory conformation, as in interphase FISH. This is accomplished by applying mechanical shear along the length of the slide, either to cells that have been fixed to the slide and then lysed, or to a solution of purified DNA. A technique known as chromosome combing is increasingly used for this purpose. The extended conformation of the chromosomes allows dramatically higher resolution – even down to a few kilobases. The preparation of fiber FISH samples, although conceptually simple, is a rather skilled art, and only specialized laboratories use the technique routinely.[33]
Q-FISH
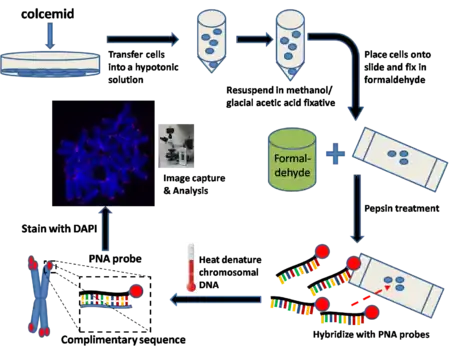
Q-FISH combines FISH with PNAs and computer software to quantify fluorescence intensity. This technique is used routinely in telomere length research.[34]
Flow-FISH
Flow-FISH uses flow cytometry to perform FISH automatically using per-cell fluorescence measurements.[35]
MA-FISH
Microfluidics-assisted FISH ( MA-FISH) uses a microfluidic flow to increase DNA hybridization efficiency, decreasing expensive FISH probe consumption and reduce the hybridization time. MA-FISH is applied for detecting the HER2 gene in breast cancer tissues.[36]
MAR-FISH
Microautoradiography FISH is a technique to combine radio-labeled substrates with conventional FISH to detect phylogenetic groups and metabolic activities simultaneously.[37]
Hybrid Fusion-FISH
Hybrid Fusion FISH uses primary additive excitation/emission combination of fluorophores to generate additional spectra through a labeling process known as dynamic optical transmission. Three primary fluorophores are able to generate a total of 7 readily detectable emission spectra as a result of combinatorial labeling using DOT. Hybrid Fusion FISH enables highly multiplexed FISH applications that are targeted within clinical oncology panels.[38][39]
MERFISH
Multiplexed error-robust fluorescence in situ hybridization[40] is a highly multiplexed version of smFISH. It uses combinatorial labeling, followed by imaging, and then error-resistant encoding[40] to capture a high number of RNA molecules and spatial localization within the cell. The capture of a large number of RNA molecules enables elucidation of gene regulatory networks, prediction of function of unannotated genes, and identification of RNA molecule distribution patterns, which correlate with their associated proteins.[41][40]
STARFISH
Starfish is a set of software tools developed to analyze data from different variations of FISH, since all variations produce the same set of data—gene expression values mapped to x and y coordinates in a cell. The software, reads a set of images, removes noise, and identifies RNA molecules. This approach has set out to define a standard analysis scheme of FISH datasets in a similar way to single-cell transcriptomics analysis.[42][43]
Medical applications
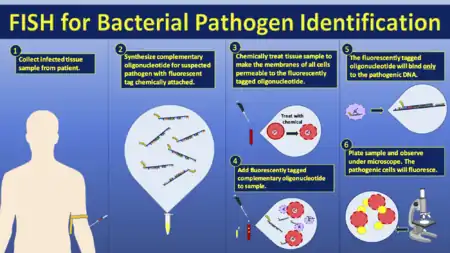
Often parents of children with a developmental disability want to know more about their child's conditions before choosing to have another child. These concerns can be addressed by analysis of the parents' and child's DNA. In cases where the child's developmental disability is not understood, the cause of it can potentially be determined using FISH and cytogenetic techniques. Examples of diseases that are diagnosed using FISH include Prader-Willi syndrome, Angelman syndrome, 22q13 deletion syndrome, chronic myelogenous leukemia, acute lymphoblastic leukemia, Cri-du-chat, Velocardiofacial syndrome, and Down syndrome. FISH on sperm cells is indicated for men with an abnormal somatic or meiotic karyotype as well as those with oligozoospermia, since approximately 50% of oligozoospermic men have an increased rate of sperm chromosome abnormalities.[44] The analysis of chromosomes 21, X, and Y is enough to identify oligozoospermic individuals at risk.[44]
In medicine, FISH can be used to form a diagnosis, to evaluate prognosis, or to evaluate remission of a disease, such as cancer. Treatment can then be specifically tailored. A traditional exam involving metaphase chromosome analysis is often unable to identify features that distinguish one disease from another, due to subtle chromosomal features; FISH can elucidate these differences. FISH can also be used to detect diseased cells more easily than standard Cytogenetic methods, which require dividing cells and requires labor and time-intensive manual preparation and analysis of the slides by a technologist. FISH, on the other hand, does not require living cells and can be quantified automatically, a computer counts the fluorescent dots present. However, a trained technologist is required to distinguish subtle differences in banding patterns on bent and twisted metaphase chromosomes. FISH can be incorporated into Lab-on-a-chip microfluidic device. This technology is still in a developmental stage but, like other lab on a chip methods, it may lead to more portable diagnostic techniques.[45][46][22]
Species identification
FISH has been extensively studied as a diagnostic technique for the identification of pathogens in the field of medical microbiology.[48] Although it has been proven to be a useful and applicable technique, it is still not widely applied in diagnostic laboratories. The short time to diagnosis (less than 2 hours) has been a major advantage compared with biochemical differentiation, but this advantage is challenged by MALDI-TOF-MS which allows the identification of a wider range of pathogens compared with biochemical differentiation techniques. Using FISH for diagnostic purposes has found its purpose when immediate species identification is needed, specifically for the investigation of blood cultures for which FISH is a cheap and easy technique for preliminary rapid diagnosis.[48]
FISH can also be used to compare the genomes of two biological species, to deduce evolutionary relationships. A similar hybridization technique is called a zoo blot. Bacterial FISH probes are often primers for the 16s rRNA region.[49][50][51]
FISH is widely used in the field of microbial ecology, to identify microorganisms. Biofilms, for example, are composed of complex multi-species bacterial organizations. Preparing DNA probes for one species and performing FISH with this probe allows one to visualize the distribution of this specific species within the biofilm.[52][47]
Comparative genomic hybridization
Comparative genomic hybridization can be described as a method that uses FISH in a parallel manner with the comparison of the hybridization strength to recall any major disruptions in the duplication process of the DNA sequences in the genome of the nucleus.[53]
Virtual karyotype
Virtual karyotyping is a clinically available alternative to FISH panels using thousands to millions of probes on a single array to detect copy number changes, genome-wide, at unprecedented resolution. Currently, this type of analysis will only detect gains and losses of chromosomal material and will not detect balanced rearrangements, such as translocations and inversions which are hallmark aberrations seen in many types of leukemia and lymphoma.[54]
Spectral karyotype
Spectral karyotyping is an image of colored chromosomes, Spectral karyotyping involves FISH using multiple forms of many types of probes with the result to see each chromosome labeled through its metaphase stage.[55]
Chromosome evolution
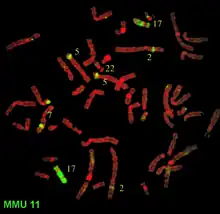
FISH can be used to study the evolution of chromosomes. Species that are related have similar chromosomes.[56]
This homology can be detected by gene or genome sequencing but also by FISH. For instance, human and chimpanzee chromosomes are very similar and FISH can demonstrate that two chimpanzee chromosomes fused to result in one human chromosome.[56]
Similarly, species that are more distantly related, have similar chromosomes but with increasing distance chromosomes tend to break and fuse and thus result in mosaic chromosomes. This can be impressively demonstrated by FISH .[56]
See also
- Chromogenic in situ hybridization (CISH)
- Eukaryotic chromosome fine structure
- G banding
- Gene mapping
- Genome evolution
- Happy mapping
- In situ hybridization
- Molecular cytogenetics
- Virtual karyotype
References
- 1 2 3 Shakoori, Abdul Rauf (10 February 2017). "Fluorescence In Situ Hybridization (FISH) and Its Applications". Chromosome Structure and Aberrations: 343–367. doi:10.1007/978-81-322-3673-3_16. ISBN 978-81-322-3671-9. PMC 7122835.
- ↑ Langer-Safer PR, Levine M, Ward DC (July 1982). "Immunological method for mapping genes on Drosophila polytene chromosomes". Proceedings of the National Academy of Sciences of the United States of America. 79 (14): 4381–4385. Bibcode:1982PNAS...79.4381L. doi:10.1073/pnas.79.14.4381. PMC 346675. PMID 6812046.
- ↑ Amann R, Fuchs BM (May 2008). "Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques". Nature Reviews. Microbiology. 6 (5): 339–348. doi:10.1038/nrmicro1888. PMID 18414500. S2CID 22498325.
- ↑ Micropatterning in Cell Biology, Part A. Elsevier. 16 January 2014. p. 294. ISBN 978-0-12-416746-9. Archived from the original on 4 November 2023. Retrieved 1 November 2023.
- ↑ Anthony SJ, St Leger JA, Pugliares K, Ip HS, Chan JM, Carpenter ZW, et al. (2012). "Emergence of fatal avian influenza in New England harbor seals". mBio. 3 (4): e00166–e00112. doi:10.1128/mBio.00166-12. PMC 3419516. PMID 22851656.
- ↑ Everitt AR, Clare S, Pertel T, John SP, Wash RS, Smith SE, et al. (March 2012). "IFITM3 restricts the morbidity and mortality associated with influenza". Nature. 484 (7395): 519–523. Bibcode:2012Natur.484..519.. doi:10.1038/nature10921. PMC 3648786. PMID 22446628.
- ↑ Louzada S, Adega F, Chaves R (2012). "Defining the sister rat mammary tumor cell lines HH-16 cl.2/1 and HH-16.cl.4 as an in vitro cell model for Erbb2". PLOS ONE. 7 (1): e29923. Bibcode:2012PLoSO...729923L. doi:10.1371/journal.pone.0029923. PMC 3254647. PMID 22253826.
- ↑ Ting DT, Lipson D, Paul S, Brannigan BW, Akhavanfard S, Coffman EJ, et al. (February 2011). "Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers". Science. 331 (6017): 593–596. Bibcode:2011Sci...331..593T. doi:10.1126/science.1200801. PMC 3701432. PMID 21233348.
- ↑ Zhang B, Arun G, Mao YS, Lazar Z, Hung G, Bhattacharjee G, et al. (July 2012). "The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult". Cell Reports. 2 (1): 111–123. doi:10.1016/j.celrep.2012.06.003. PMC 3408587. PMID 22840402.
- ↑ Lee K, Kunkeaw N, Jeon SH, Lee I, Johnson BH, Kang GY, et al. (June 2011). "Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity". RNA. 17 (6): 1076–1089. doi:10.1261/rna.2701111. PMC 3096040. PMID 21518807.
- 1 2 3 Bernasconi B, Karamitopoulou-Diamantis E, Karamitopolou-Diamantiis E, Tornillo L, Lugli A, Di Vizio D, et al. (April 2008). "Chromosomal instability in gastric mucosa-associated lymphoid tissue lymphomas: a fluorescent in situ hybridization study using a tissue microarray approach". Human Pathology. 39 (4): 536–542. doi:10.1016/j.humpath.2007.08.009. PMID 18234275.
- 1 2 3 Cui C, Shu W, Li P (2016). "Fluorescence In situ Hybridization: Cell-Based Genetic Diagnostic and Research Applications". Frontiers in Cell and Developmental Biology. 4: 89. doi:10.3389/fcell.2016.00089. PMC 5011256. PMID 27656642.
- 1 2 Orjalo AV, Johansson HE (2016-01-01). "Stellaris® RNA Fluorescence in Situ Hybridization for the Simultaneous Detection of Immature and Mature Long Noncoding RNAs in Adherent Cells". In Feng Y, Zhang L (eds.). Long Non-Coding RNAs. Methods in Molecular Biology. Vol. 1402. Springer New York. pp. 119–134. doi:10.1007/978-1-4939-3378-5_10. ISBN 9781493933761. PMID 26721487.
- ↑ "Bacterial Artificial Chromosome (BAC)". Genome.gov. Archived from the original on 11 July 2023. Retrieved 11 November 2023.
- 1 2 3 4 Young AP, Jackson DJ, Wyeth RC (2020-03-19). "A technical review and guide to RNA fluorescence in situ hybridization". PeerJ. 8: e8806. doi:10.7717/peerj.8806. PMC 7085896. PMID 32219032.
- ↑ Haroon MF, Skennerton CT, Steen JA, Lachner N, Hugenholtz P, Tyson GW (2013). "In-Solution Fluorescence in Situ Hybridization and Fluorescence-Activated Cell Sorting for Single Cell and Population Genome Recovery". Microbial Metagenomics, Metatranscriptomics, and Metaproteomics. Methods in Enzymology. Vol. 531. pp. 3–19. doi:10.1016/B978-0-12-407863-5.00001-0. ISBN 9780124078635. PMID 24060113.
- ↑ Xie F, Timme KA, Wood JR (May 2018). "Using Single Molecule mRNA Fluorescent in Situ Hybridization (RNA-FISH) to Quantify mRNAs in Individual Murine Oocytes and Embryos". Scientific Reports. 8 (1): 7930. Bibcode:2018NatSR...8.7930X. doi:10.1038/s41598-018-26345-0. PMC 5962540. PMID 29785002.
- ↑ Oliveira VC, Carrara RC, Simoes DL, Saggioro FP, Carlotti CG, Covas DT, Neder L (August 2010). "Sudan Black B treatment reduces autofluorescence and improves resolution of in situ hybridization specific fluorescent signals of brain sections". Histology and Histopathology. 25 (8): 1017–1024. doi:10.14670/HH-25.1017. PMID 20552552.
- ↑ "DNA Precipitation and Hybridization (FISH)" (PDF). cancer.gov. Archived (PDF) from the original on 11 April 2021. Retrieved 3 November 2023.
- ↑ Chrzanowska, Natalia Magdalena; Kowalewski, Janusz; Lewandowska, Marzena Anna (January 2020). "Use of Fluorescence In Situ Hybridization (FISH) in Diagnosis and Tailored Therapies in Solid Tumors". Molecules. 25 (8): 1864. doi:10.3390/molecules25081864. ISSN 1420-3049. Archived from the original on 2023-05-06. Retrieved 2023-11-08.
- ↑ Veselinyová, Dominika; Mašlanková, Jana; Kalinová, Katarina; Mičková, Helena; Mareková, Mária; Rabajdová, Miroslava (24 June 2021). "Selected In Situ Hybridization Methods: Principles and Application". Molecules. 26 (13): 3874. doi:10.3390/molecules26133874. ISSN 1420-3049. Archived from the original on 13 November 2023. Retrieved 12 November 2023.
- 1 2 3 Bishop, R. (1 March 2010). "Applications of fluorescence in situ hybridization (FISH) in detecting genetic aberrations of medical significance". Bioscience Horizons. 3 (1): 85–95. doi:10.1093/biohorizons/hzq009. Archived from the original on 29 October 2023. Retrieved 4 November 2023.
- ↑ Young, Alexander P.; Jackson, Daniel J.; Wyeth, Russell C. (19 March 2020). "A technical review and guide to RNA fluorescence in situ hybridization". PeerJ. 8: e8806. doi:10.7717/peerj.8806. ISSN 2167-8359. Archived from the original on 6 October 2023. Retrieved 11 November 2023.
- ↑ Kato, Takamitsu A. (2023). "FISH with Whole Chromosome Painting Probes". Methods in Molecular Biology (Clifton, N.J.). 2519: 99–104. doi:10.1007/978-1-0716-2433-3_11. ISSN 1940-6029. Archived from the original on 2022-09-08. Retrieved 2023-11-02.
- ↑ Ried, T (1 September 1998). "Chromosome painting: a useful art". Human Molecular Genetics. 7 (10): 1619–1626. doi:10.1093/hmg/7.10.1619. Archived from the original on 5 June 2018. Retrieved 2 November 2023.
- ↑ Yanagi, M.; Shinjo, K.; Takeshita, A.; Tobita, T.; Yano, K.; Kobayashi, M.; Terasaki, H.; Naoe, T.; Ohnishi, K.; Ohno, R. (April 1999). "Simple and reliably sensitive diagnosis and monitoring of Philadelphia chromosome-positive cells in chronic myeloid leukemia by interphase fluorescence in situ hybridization of peripheral blood cells". Leukemia. 13 (4): 542–552. doi:10.1038/sj.leu.2401383. ISSN 1476-5551. Archived from the original on 2023-11-07. Retrieved 2023-11-04.
- ↑ Jensen, Ellen (August 2014). "Technical Review: In Situ Hybridization". The Anatomical Record. 297 (8): 1349–1353. doi:10.1002/ar.22944. ISSN 1932-8486. Archived from the original on 2023-05-19. Retrieved 2023-11-11.
- ↑ Chen J, McSwiggen D, Ünal E (May 2018). "Single Molecule Fluorescence In Situ Hybridization (smFISH) Analysis in Budding Yeast Vegetative Growth and Meiosis". Journal of Visualized Experiments (135). doi:10.3791/57774. PMC 6101419. PMID 29889208.
- ↑ Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S (October 2008). "Imaging individual mRNA molecules using multiple singly labeled probes". Nature Methods. 5 (10): 877–879. doi:10.1038/nmeth.1253. PMC 3126653. PMID 18806792.
- ↑ Biosearch Technologies Signs Exclusive License for Single Molecule FISH Technologies from UMDNJ Archived 2016-04-02 at the Wayback Machine. biosearchtech.com
- ↑ Cagir B, Gelmann A, Park J, Fava T, Tankelevitch A, Bittner EW, et al. (December 1999). "Guanylyl cyclase C messenger RNA is a biomarker for recurrent stage II colorectal cancer". Annals of Internal Medicine. 131 (11): 805–812. doi:10.7326/0003-4819-131-11-199912070-00024. PMID 10610624.
- ↑ Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E (August 2004). "Multiplex detection of RNA expression in Drosophila embryos". Science. 305 (5685): 846. doi:10.1126/science.1099247. PMID 15297669. S2CID 26313219.
- ↑ Heiskanen M, Kallioniemi O, Palotie A (March 1996). "Fiber-FISH: experiences and a refined protocol". Genetic Analysis. 12 (5–6): 179–184. doi:10.1016/S1050-3862(96)80004-0. PMID 8740834.
- ↑ Iourov, Ivan Y. (2017). "Quantitative Fluorescence In Situ Hybridization (QFISH)". Cancer Cytogenetics. Methods in Molecular Biology (Clifton, N.J.). Vol. 1541. pp. 143–149. doi:10.1007/978-1-4939-6703-2_13. ISBN 978-1-4939-6701-8. ISSN 1940-6029. PMID 27910021. Archived from the original on 4 November 2022. Retrieved 28 October 2023.
- ↑ Baerlocher, Gabriela M.; Vulto, Irma; de Jong, Gary; Lansdorp, Peter M. (2006). "Flow cytometry and FISH to measure the average length of telomeres (flow FISH)". Nature Protocols. 1 (5): 2365–2376. doi:10.1038/nprot.2006.263. ISSN 1750-2799. PMID 17406480. S2CID 20463557. Archived from the original on 3 June 2023. Retrieved 28 October 2023.
- ↑ Nguyen HT, Trouillon R, Matsuoka S, Fiche M, de Leval L, Bisig B, Gijs MA (January 2017). "Microfluidics-assisted fluorescence in situ hybridization for advantageous human epidermal growth factor receptor 2 assessment in breast cancer". Laboratory Investigation; A Journal of Technical Methods and Pathology. 97 (1): 93–103. doi:10.1038/labinvest.2016.121. PMID 27892928.
- ↑ Okabe S, Kindaichi T, Ito T (2004). "MAR-FISH—An Ecophysiological Approach to Link Phylogenetic Affiliation and In Situ Metabolic Activity of Microorganisms at a Single-Cell Resolution". Microbes and Environments. 19 (2): 83–98. doi:10.1264/jsme2.19.83.
- ↑ "HYBRID FUSION FISH — Prevnos". 20 November 2022. Archived from the original on 20 November 2022. Retrieved 28 October 2023.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ↑ Harris, Mohammed (15 December 2017). "Hybrid Fusion FISH™ vs Next-Generation Sequencing: A Comparison of Diagnostic Values Between…". Medium. Archived from the original on 15 August 2020. Retrieved 3 November 2023.
- 1 2 3 Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X (April 2015). "RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells". Science. 348 (6233): aaa6090. doi:10.1126/science.aaa6090. PMC 4662681. PMID 25858977.
- ↑ Liu, Jonathan; Tran, Vanessa; Vemuri, Venkata Naga Pranathi; Byrne, Ashley; Borja, Michael; Kim, Yang Joon; Agarwal, Snigdha; Wang, Ruofan; Awayan, Kyle; Murti, Abhishek; Taychameekiatchai, Aris; Wang, Bruce; Emanuel, George; He, Jiang; Haliburton, John; Oliveira Pisco, Angela; Neff, Norma F. (January 2023). "Concordance of MERFISH spatial transcriptomics with bulk and single-cell RNA sequencing". Life Science Alliance. 6 (1): e202201701. doi:10.26508/lsa.202201701. ISSN 2575-1077. PMID 36526371.
- ↑ Perkel JM (August 2019). "Starfish enterprise: finding RNA patterns in single cells". Nature. 572 (7770): 549–551. Bibcode:2019Natur.572..549P. doi:10.1038/d41586-019-02477-9. PMID 31427807. S2CID 201064966.
- ↑ Cockroft, Nicholas T.; Cheng, Xiaolin; Fuchs, James R. (25 November 2019). "STarFish: A Stacked Ensemble Target Fishing Approach and its Application to Natural Products". Journal of chemical information and modeling. 59 (11): 4906–4920. doi:10.1021/acs.jcim.9b00489. ISSN 1549-9596. Archived from the original on 7 November 2023. Retrieved 5 November 2023.
- 1 2 Sarrate Z, Vidal F, Blanco J (April 2010). "Role of sperm fluorescent in situ hybridization studies in infertile patients: indications, study approach, and clinical relevance". Fertility and Sterility. 93 (6): 1892–1902. doi:10.1016/j.fertnstert.2008.12.139. PMID 19254793.
- ↑ Kurz CM, Moosdijk SV, Thielecke H, Velten T (2011). "Towards a cellular multi-parameter analysis platform: Fluorescence in situ hybridization (FISH) on microhole-array chips". 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol. 2011. pp. 8408–8411. doi:10.1109/IEMBS.2011.6092074. ISBN 978-1-4577-1589-1. PMID 22256298. S2CID 4955677.
- ↑ Dill K, Liu R, Grodzinsky P, eds. (2008). Microarrays: Preparation, Microfluidics, Detection Methods, and Biological Applications. Springer. p. 323. ISBN 978-0387727165.
- 1 2 Barbosa, Ana; Miranda, Sónia; Azevedo, Nuno F.; Cerqueira, Laura; Azevedo, Andreia S. (2023). "Imaging biofilms using fluorescence in situ hybridization: seeing is believing". Frontiers in Cellular and Infection Microbiology. 13: 1195803. doi:10.3389/fcimb.2023.1195803. ISSN 2235-2988. Archived from the original on 2023-11-01. Retrieved 2023-11-11.
- 1 2 Frickmann H, Zautner AE, Moter A, Kikhney J, Hagen RM, Stender H, Poppert S (May 2017). "Fluorescence in situ hybridization (FISH) in the microbiological diagnostic routine laboratory: a review". Critical Reviews in Microbiology. 43 (3): 263–293. doi:10.3109/1040841X.2016.1169990. PMID 28129707. S2CID 25252460.
- ↑ Khan, Firdos Alam (20 September 2011). Biotechnology Fundamentals. CRC Press. p. 563. ISBN 978-1-4398-2009-4. OCLC 708544338. Archived from the original on 7 November 2023. Retrieved 4 November 2023.
- ↑ Hugenholtz, Philip; Tyson, Gene W.; Blackall, Linda L. (2002). "Design and Evaluation of 16S rRNA-Targeted Oligonucleotide Probes for Fluorescence In Situ Hybridization". Gene Probes: Principles and Protocols. Humana Press. pp. 29–42. ISBN 978-1-59259-238-8. Archived from the original on 2021-09-29. Retrieved 2023-11-12.
- ↑ Wienberg, J. (12 November 2004). "Fluorescence in situ hybridization to chromosomes as a tool to understand human and primate genome evolution". Cytogenetic and Genome Research. 108 (1–3): 139–160. doi:10.1159/000080811. ISSN 1424-8581. Archived from the original on 13 November 2023. Retrieved 12 November 2023.
- ↑ Bottari, Benedetta; Ercolini, Danilo; Gatti, Monica; Neviani, Erasmo (December 2006). "Application of FISH technology for microbiological analysis: current state and prospects". Applied Microbiology and Biotechnology. 73 (3): 485–494. doi:10.1007/s00253-006-0615-z. ISSN 0175-7598. PMID 17051413. S2CID 24247848. Archived from the original on 18 June 2022. Retrieved 30 October 2023.
- ↑ "Comparative Genomic Hybridization". McGraw-Hill Dictionary of Scientific and Technical Terms. Archived from the original on May 30, 2020. Retrieved September 19, 2013.
- ↑ Wang, Tian-Li; Maierhofer, Christine; Speicher, Michael R.; Lengauer, Christoph; Vogelstein, Bert; Kinzler, Kenneth W.; Velculescu, Victor E. (10 December 2002). "Digital karyotyping". Proceedings of the National Academy of Sciences. 99 (25): 16156–16161. Bibcode:2002PNAS...9916156W. doi:10.1073/pnas.202610899. ISSN 0027-8424. PMC 138581. PMID 12461184.
- ↑ "Spectral Karyotype (SKY)". Genome.gov. Archived from the original on 14 April 2023. Retrieved 29 October 2023.
- 1 2 3 4 Ferguson-Smith MA, Pereira JC, Borges A, Kasai F (October 2022). "Observations on chromosome-specific sequencing for the construction of cross-species chromosome homology maps and its resolution of human:alpaca homology". Molecular Cytogenetics. 15 (1): 44. doi:10.1186/s13039-022-00622-0. PMID 36207754.
Further reading
- Pernthaler A, Pernthaler J, Amann R (June 2002). "Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria". Applied and Environmental Microbiology. 68 (6): 3094–3101. Bibcode:2002ApEnM..68.3094P. doi:10.1128/AEM.68.6.3094-3101.2002. PMC 123953. PMID 12039771.
- Wagner M, Horn M, Daims H (June 2003). "Fluorescence in situ hybridisation for the identification and characterisation of prokaryotes". Current Opinion in Microbiology. 6 (3): 302–309. doi:10.1016/S1369-5274(03)00054-7. PMID 12831908.
- Carthy JD (1965). Viewpoints In Biology. England: Butterworth & Co. p. 66.
- Richardson, Shivanand O.; Huibers, Manon M. H.; de Weger, Roel A.; de Leng, Wendy W. J.; Hinrichs, John W. J.; Meijers, Ruud W. J.; Willems, Stefan M.; Peeters, Ton L. M. G. (17 June 2019). "One-fits-all pretreatment protocol facilitating Fluorescence In Situ Hybridization on formalin-fixed paraffin-embedded, fresh frozen and cytological slides". Molecular Cytogenetics. 12 (1): 27. doi:10.1186/s13039-019-0442-4. ISSN 1755-8166. Archived from the original on 21 September 2023. Retrieved 2 November 2023.
- Shah, Jyotsna S.; Ramasamy, Ranjan (May 2022). "Fluorescence In Situ Hybridization (FISH) Tests for Identifying Protozoan and Bacterial Pathogens in Infectious Diseases". Diagnostics. 12 (5): 1286. doi:10.3390/diagnostics12051286. ISSN 2075-4418. Archived from the original on 2023-08-01. Retrieved 2023-11-12.
External links
- Fluorescent+in+Situ+Hybridization at the US National Library of Medicine Medical Subject Headings (MeSH)
- Information on fiber FISH from the Olympus Corporation
- A guide to fiber FISH Archived 2010-11-25 at the Wayback Machine from Octavian Henegariu
- Fibre FISH protocol Archived 2006-10-23 at the Wayback Machine from the Human Genome Project at the Sanger Centre
- CARD-FISH, BioMineWiki Archived 2020-07-28 at the Wayback Machine
- Preparation of Complex DNA Probe Sets for 3D FISH with up to Six Different Fluorochromes Archived 2008-06-19 at the Wayback Machine
- FISH technical notes and protocols from GeneDetect.com Archived 2021-01-19 at the Wayback Machine
- Fluorescence in situ Hybridization Photos of bacteria Archived 2015-02-05 at the Wayback Machine
- Rational design of polynucleotide probe mixes to identify particular genes in defined taxa: www.dnaBaser.com/PolyPro