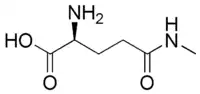
Γ-Glutamylmethylamide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
N-Methyl-L-glutamine | |
| Systematic IUPAC name
(2S)-2-Amino-5-(methylamino)-5-oxopentanoic acid | |
| Other names
Metheanine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C6H12N2O3 |
| Molar mass | 160.17 g/mol |
| Density | 1.211 g/mL |
| Boiling point | 427.5 °C (801.5 °F; 700.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
γ-Glutamylmethylamide (gamma-glutamylmethylamide, abbrev. GMA, synonyms N-methyl-L-glutamine, metheanine) is an amino acid analog of the proteinogenic amino acids L-glutamic acid and L-glutamine, found primarily in plant and fungal species; simply speaking, it is L-glutamine methylated on the amide nitrogen. It is an identified important biosynthetic intermediate allowing bacteria (e.g., methanotrophs) use of methylated amines as carbon and nitrogen source for growth (and so of significant biotechnological interest).[1][2][3] Like its close relative theanine, it is a pharmacologically active constituent of green tea, with preliminary evidence for at least comparable activity to theanine as a hypotensive.[4]
See also
References
- ↑ Chen, Y; Scanlan, J; Song, L; Crombie, A; Rahman, MT; Schäfer, H; Murrell, JC (2010). "γ-Glutamylmethylamide is an essential intermediate in the metabolism of methylamine by Methylocella silvestris". Appl Environ Microbiol. 76 (13): 4530–4537. Bibcode:2010ApEnM..76.4530C. doi:10.1128/AEM.00739-10. PMC 2897447. PMID 20472738.
- ↑ Wischer, D; Kumaresan, D; Johnston, A; El Khawand, M; Stephenson, J; Hillebrand-Voiculescu, AM; Chen, Y; Colin Murrell, J (2014). "Bacterial metabolism of methylated amines and identification of novel methylotrophs in Movile Cave". ISME J. 9 (1): 195–206. doi:10.1038/ismej.2014.102. PMC 4274414. PMID 25050523.
- ↑ Xu, L; Gao, G; Wengen, C; Xu, J; Zhao, L; Shi, H; Zhang, X (2014). "Enzymatic synthesis of γ-glutamylmethylamide from glutamic acid γ-methyl ester and methylamine catalyzed by Escherichia coli having γ-glutamyltranspeptidase activity". Appl Biochem Biotechnol. 173 (4): 851–6. doi:10.1007/s12010-014-0877-3. PMID 24733529. S2CID 19699395.
- ↑ Yokogoshi H, Kobayashi M. (1998). "Hypotensive effect of gamma-glutamylmethylamide in spontaneously hypertensive rats". Life Sci. 62 (12): 1065–8. doi:10.1016/S0024-3205(98)00029-0. PMID 9519808.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.