Globoidnan A
 | |
| Names | |
|---|---|
| Preferred IUPAC name
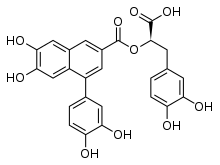
(2R)-3-(3,4-Dihydroxyphenyl)-2-{[4-(3,4-dihydroxyphenyl)-6,7-dihydroxynaphthalene-2-carbonyl]oxy}propanoic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C26H20O10 |
| Molar mass | 492.436 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Globoidnan A is a lignan found in Eucalyptus globoidea, a tree native to Australia.
The molecule has been found to weakly inhibit the action of HIV integrase (IC50 = 0.64 μM) in vitro.[1] HIV integrase is an enzyme which is responsible for the introduction of HIV viral DNA into a host's cellular DNA. It is not known that globoidnan A inhibits the action of other retroviral integrases.
References
- ↑ Ovenden SP, Yu J, Wan SS, et al. (2004). "Globoidnan A: a lignan from Eucalyptus globoidea inhibits HIV integrase". Phytochemistry. 65 (24): 3255–59. doi:10.1016/j.phytochem.2004.10.006. PMID 15561191.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.