H3K9me2
H3K9me2 is an epigenetic modification to the DNA packaging protein Histone H3. It is a mark that indicates the di-methylation at the 9th lysine residue of the histone H3 protein. H3K9me2 is strongly associated with transcriptional repression.[1][2][3] H3K9me2 levels are higher at silent compared to active genes in a 10kb region surrounding the transcriptional start site.[4] H3K9me2 represses gene expression both passively, by prohibiting acetylation[5] and therefore binding of RNA polymerase or its regulatory factors, and actively, by recruiting transcriptional repressors.[6][7] H3K9me2 has also been found in megabase blocks, termed Large Organised Chromatin K9 domains (LOCKS), which are primarily located within gene-sparse regions but also encompass genic and intergenic intervals.[8][9][10][11] Its synthesis is catalyzed by G9a, G9a-like protein, and PRDM2.[1][3][12] H3K9me2 can be removed by a wide range of histone lysine demethylases (KDMs) including KDM1, KDM3, KDM4 and KDM7 family members.[13][6] H3K9me2 is important for various biological processes including cell lineage commitment,[10][14] the reprogramming of somatic cells to induced pluripotent stem cells,[15] regulation of the inflammatory response,[16][17] and addiction to drug use.[2][18][19][20]
Nomenclature
H3K9me2 indicates dimethylation of lysine 9 on histone H3 protein subunit:[21]
| Abbr. | Meaning |
| H3 | H3 family of histones |
| K | standard abbreviation for lysine |
| 9 | position of amino acid residue
(counting from N-terminus) |
| me | methyl group |
| 2 | number of methyl groups added |
Lysine Methylation
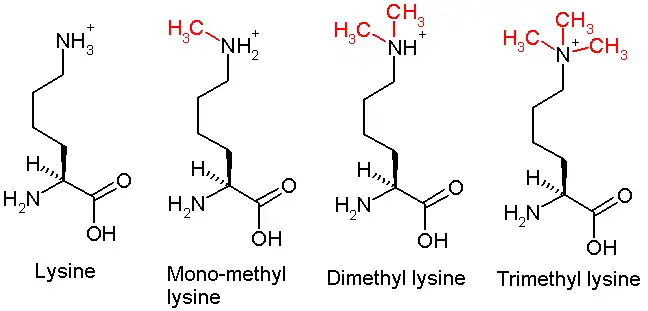
This diagram shows the progressive methylation of a lysine residue. The di-methylation denotes the methylation present in H3K9me2.
Understanding histone modifications
The genomic DNA of eukaryotic cells is wrapped around special protein molecules known as histones. The complexes formed by the looping of the DNA are known as chromatin. The basic structural unit of chromatin is the nucleosome: this consists of the core octamer of histones (H2A, H2B, H3 and H4) as well as a linker histone and about 180 base pairs of DNA. These core histones are rich in lysine and arginine residues. The carboxyl (C) terminal end of these histones contribute to histone-histone interactions, as well as histone-DNA interactions. The amino (N) terminal charged tails are the site of the post-translational modifications, such as the one seen in H3K9me2.[22][23]
Epigenetic implications
The post-translational modification of histone tails by either histone modifying complexes or chromatin remodelling complexes are interpreted by the cell and lead to complex, combinatorial transcriptional output. It is thought that a histone code dictates the expression of genes by a complex interaction between the histones in a particular region.[24] The current understanding and interpretation of histones comes from two large scale projects: ENCODE and the Epigenomic roadmap.[25] The purpose of the epigenomic study was to investigate epigenetic changes across the entire genome. This led to chromatin states which define genomic regions by grouping the interactions of different proteins and/or histone modifications together. Chromatin states were investigated in Drosophila cells by looking at the binding location of proteins in the genome. Use of chromatin immunoprecipitation (ChIP)-sequencing revealed regions in the genome characterised by different banding.[26] Different developmental stages were profiled in Drosophila as well, an emphasis was placed on histone modification relevance.[27] A look in to the data obtained led to the definition of chromatin states based on histone modifications.[28] Certain modifications were mapped and enrichment was seen to localize in certain genomic regions. Five core histone modifications were found with each respective one being linked to various cell functions.
- H3K4me3-promoters
- H3K4me1- primed enhancers
- H3K36me3-gene bodies
- H3K27me3-polycomb repression
- H3K9me3-heterochromatin
- H3K9me2-facultative heterochromatin
The human genome was annotated with chromatin states. These annotated states can be used as new ways to annotate a genome independently of the underlying genome sequence. This independence from the DNA sequence enforces the epigenetic nature of histone modifications. Chromatin states are also useful in identifying regulatory elements that have no defined sequence, such as enhancers. This additional level of annotation allows for a deeper understanding of cell specific gene regulation.[29]
Clinical significance
Addiction
Chronic addictive drug exposure results in ΔFosB-mediated repression of G9a and reduced H3K9 dimethylation in the nucleus accumbens, which in turn causes dendritic arborization, altered synaptic protein expression, and increased drug seeking behavior.[2][18] In contrast, accumbal G9a hyperexpression results in markedly increased H3K9 dimethylation and blocks the induction of this neural and behavioral plasticity by chronic drug use,[2][19][20][30] which occurs via H3K9me2-mediated repression of transcription factors for ΔFosB and H3K9me2-mediated repression of various ΔFosB transcriptional targets (e.g., CDK5).[2][18][19] Due to the involvement of H3K9me2 in these feedback loops and the central pathophysiological role of ΔFosB overexpression as the mechanistic trigger for addiction,[2][31] the reduction of accumbal H3K9me2 following repeated drug exposure directly mediates the development of drug addictions.[18][19]
Friedreich's ataxia
R-loop's are found with H3K9me2 mark at FXN in Friedreich's ataxia cells.[32]
Cardiovascular disease
H3K9me2 is present at a subset of cardiovascular disease-associated gene promoters in vascular smooth muscle cells[16] to block binding of NFκB and AP-1 (activator protein-1) transcription factors.[16] Reduced levels of H3K9me2 have been observed in vascular smooth muscle cells from human atherosclerotic lesions compared to healthy aortic tissue in patients.[33] Vascular smooth muscle cells from diabetic patients display reduced levels of H3K9me2 compared to non-diabetic controls; it has therefore been suggested that dysregulation of H3K9me2 might underlie the vascular complications associated with diabetes.[34][35] Loss of H3K9me2 in vascular smooth muscle cells exacerbates upregulation of a subset of cardiovascular disease-associated genes in vascular disease models.[16][34][36]
Methods
Histone modifications, including H3K9me2, can be detected using a variety of methods:
- Chromatin Immunoprecipitation Sequencing (ChIP-sequencing) measures the amount of DNA enrichment once bound to a targeted protein and immunoprecipitated. It results in good optimization and is used in vivo to reveal DNA-protein binding occurring in cells. ChIP-Seq can be used to identify and quantify various DNA fragments for different histone modifications along a genomic region.[37]
- CUT&RUN(Cleavage Under Targets and Release Using Nuclease). In CUT&RUN, targeted DNA-protein complexes are isolated directly from the cell nucleus rather than following a precipitation step. To perform CUT&RUN, a specific antibody to the DNA-binding protein of interest and ProtA-MNase is added to permeabilised cells. MNase is tethered to the protein of interest through the ProtA-antibody interaction and MNase cleaves the surrounding, unprotected DNA to release protein-DNA complexes, which can then be isolated and sequenced.[38][39] CUT&RUN is reported to give a much higher signal to noise ratio compared to traditional ChIP. CUT&RUN therefore requires one tenth of the sequencing depth of ChIP and permits genomic mapping of histone modifications and transcription factors using extremely low cell numbers.[40][38][39]
- Modification-specific intracellular antibody probes. Sensitive fluorescent genetically encoded histone modification-specific intracellular antibody (mintbody) probes can be used to monitor changes in histone modifications in living cells.[41]
See also
- Histone methylation
- Histone methyltransferase
- Methyllysine
References
- 1 2 "H3K9me2". HIstome: The Histone Infobase. Retrieved 8 June 2018.
- 1 2 3 4 5 6 Robison AJ, Nestler EJ (October 2011). "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews. Neuroscience. 12 (11): 623–37. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
Figure 4: Epigenetic basis of drug regulation of gene expression - 1 2 Nestler EJ (August 2015). "Role of the Brain's Reward Circuitry in Depression: Transcriptional Mechanisms". International Review of Neurobiology. 124: 151–70. doi:10.1016/bs.irn.2015.07.003. PMC 4690450. PMID 26472529.
Chronic social defeat stress decreases expression of G9a and GLP (G9a-like protein), two histone methyltransferases that catalyze the dimethylation of Lys9 of histone H3 (H3K9me2) (Covington et al., 2011), a mark associated with gene repression.
- ↑ Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. (May 2007). "High-resolution profiling of histone methylations in the human genome". Cell. 129 (4): 823–37. doi:10.1016/j.cell.2007.05.009. PMID 17512414.
- ↑ Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, et al. (July 2008). "Combinatorial patterns of histone acetylations and methylations in the human genome". Nature Genetics. 40 (7): 897–903. doi:10.1038/ng.154. PMC 2769248. PMID 18552846.
- 1 2 Shinkai Y, Tachibana M (April 2011). "H3K9 methyltransferase G9a and the related molecule GLP". Genes & Development. 25 (8): 781–8. doi:10.1101/gad.2027411. PMC 3078703. PMID 21498567.
- ↑ Zhang T, Termanis A, Özkan B, Bao XX, Culley J, de Lima Alves F, et al. (April 2016). "G9a/GLP Complex Maintains Imprinted DNA Methylation in Embryonic Stem Cells". Cell Reports. 15 (1): 77–85. doi:10.1016/j.celrep.2016.03.007. PMC 4826439. PMID 27052169.
- ↑ Filion GJ, van Steensel B (January 2010). "Reassessing the abundance of H3K9me2 chromatin domains in embryonic stem cells". Nature Genetics. 42 (1): 4, author reply 5–6. doi:10.1038/ng0110-4. PMID 20037608.
- ↑ McDonald OG, Wu H, Timp W, Doi A, Feinberg AP (July 2011). "Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition". Nature Structural & Molecular Biology. 18 (8): 867–74. doi:10.1038/nsmb.2084. PMC 3150339. PMID 21725293.
- 1 2 Wen B, Wu H, Shinkai Y, Irizarry RA, Feinberg AP (February 2009). "Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells". Nature Genetics. 41 (2): 246–50. doi:10.1038/ng.297. PMC 2632725. PMID 19151716.
- ↑ Jørgensen HF, Fisher AG (March 2009). "LOCKing in Cellular Potential". Cell Stem Cell. 4 (3): 192–4. doi:10.1016/j.stem.2009.02.007. PMID 19265653.
- ↑ "Histone-lysine N-methyltransferase, H3 lysine-9 specific 3". HIstome: The Histone Infobase. Retrieved 8 June 2018.
- ↑ Cloos PA, Christensen J, Agger K, Helin K (May 2008). "Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease". Genes & Development. 22 (9): 1115–40. doi:10.1101/gad.1652908. PMC 2732404. PMID 18451103.
- ↑ Chen X, Skutt-Kakaria K, Davison J, Ou YL, Choi E, Malik P, et al. (November 2012). "G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment". Genes & Development. 26 (22): 2499–511. doi:10.1101/gad.200329.112. PMC 3505820. PMID 23105005.
- ↑ Rodriguez-Madoz JR, San Jose-Eneriz E, Rabal O, Zapata-Linares N, Miranda E, Rodriguez S, et al. (2017). "Reversible dual inhibitor against G9a and DNMT1 improves human iPSC derivation enhancing MET and facilitating transcription factor engagement to the genome". PLOS ONE. 12 (12): e0190275. Bibcode:2017PLoSO..1290275R. doi:10.1371/journal.pone.0190275. PMC 5744984. PMID 29281720.
- 1 2 3 4 Harman JL, Dobnikar L, Chappell J, Stokell BG, Dalby A, Foote K, et al. (November 2019). "Epigenetic Regulation of Vascular Smooth Muscle Cells by Histone H3 Lysine 9 Dimethylation Attenuates Target Gene-Induction by Inflammatory Signaling". Arteriosclerosis, Thrombosis, and Vascular Biology. 39 (11): 2289–2302. doi:10.1161/ATVBAHA.119.312765. PMC 6818986. PMID 31434493.
- ↑ Fang TC, Schaefer U, Mecklenbrauker I, Stienen A, Dewell S, Chen MS, et al. (April 2012). "Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response". The Journal of Experimental Medicine. 209 (4): 661–9. doi:10.1084/jem.20112343. PMC 3328357. PMID 22412156.
- 1 2 3 4 Nestler EJ (January 2014). "Epigenetic mechanisms of drug addiction". Neuropharmacology. 76 Pt B: 259–68. doi:10.1016/j.neuropharm.2013.04.004. PMC 3766384. PMID 23643695.
- 1 2 3 4 Biliński P, Wojtyła A, Kapka-Skrzypczak L, Chwedorowicz R, Cyranka M, Studziński T (2012). "Epigenetic regulation in drug addiction". Annals of Agricultural and Environmental Medicine. 19 (3): 491–6. PMID 23020045.
- 1 2 Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, et al. (April 2013). "Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation". Nature Neuroscience. 16 (4): 434–40. doi:10.1038/nn.3354. PMC 3609040. PMID 23475113.
- ↑ Huang, Suming; Litt, Michael D.; Ann Blakey, C. (2015). Epigenetic Gene Expression and Regulation. pp. 21–38. ISBN 9780127999586.
- ↑ Ruthenburg AJ, Li H, Patel DJ, Allis CD (December 2007). "Multivalent engagement of chromatin modifications by linked binding modules". Nature Reviews. Molecular Cell Biology. 8 (12): 983–94. doi:10.1038/nrm2298. PMC 4690530. PMID 18037899.
- ↑ Kouzarides T (February 2007). "Chromatin modifications and their function". Cell. 128 (4): 693–705. doi:10.1016/j.cell.2007.02.005. PMID 17320507.
- ↑ Jenuwein T, Allis CD (August 2001). "Translating the histone code". Science. 293 (5532): 1074–80. doi:10.1126/science.1063127. PMID 11498575. S2CID 1883924.
- ↑ Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, et al. (The ENCODE Project Consortium) (June 2007). "Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project". Nature. 447 (7146): 799–816. Bibcode:2007Natur.447..799B. doi:10.1038/nature05874. PMC 2212820. PMID 17571346.
- ↑ Filion GJ, van Bemmel JG, Braunschweig U, Talhout W, Kind J, Ward LD, et al. (October 2010). "Systematic protein location mapping reveals five principal chromatin types in Drosophila cells". Cell. 143 (2): 212–24. doi:10.1016/j.cell.2010.09.009. PMC 3119929. PMID 20888037.
- ↑ Roy S, Ernst J, Kharchenko PV, Kheradpour P, Negre N, Eaton ML, et al. (modENCODE Consortium) (December 2010). "Identification of functional elements and regulatory circuits by Drosophila modENCODE". Science. 330 (6012): 1787–97. Bibcode:2010Sci...330.1787R. doi:10.1126/science.1198374. PMC 3192495. PMID 21177974.
- ↑ Kharchenko PV, Alekseyenko AA, Schwartz YB, Minoda A, Riddle NC, Ernst J, et al. (March 2011). "Comprehensive analysis of the chromatin landscape in Drosophila melanogaster". Nature. 471 (7339): 480–5. Bibcode:2011Natur.471..480K. doi:10.1038/nature09725. PMC 3109908. PMID 21179089.
- ↑ Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, et al. (Roadmap Epigenomics Consortium) (February 2015). "Integrative analysis of 111 reference human epigenomes". Nature. 518 (7539): 317–30. Bibcode:2015Natur.518..317.. doi:10.1038/nature14248. PMC 4530010. PMID 25693563.
- ↑ Whalley K (December 2014). "Psychiatric disorders: a feat of epigenetic engineering". Nature Reviews. Neuroscience. 15 (12): 768–9. doi:10.1038/nrn3869. PMID 25409693.
- ↑ Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse. 40 (6): 428–37. doi:10.3109/00952990.2014.933840. PMID 25083822. S2CID 19157711.
- ↑ Richard P, Manley JL (October 2017). "R Loops and Links to Human Disease". Journal of Molecular Biology. 429 (21): 3168–3180. doi:10.1016/j.jmb.2016.08.031. PMC 5478472. PMID 27600412.
- ↑ Greißel A, Culmes M, Napieralski R, Wagner E, Gebhard H, Schmitt M, et al. (August 2015). "Alternation of histone and DNA methylation in human atherosclerotic carotid plaques". Thrombosis and Haemostasis. 114 (2): 390–402. doi:10.1160/TH14-10-0852. PMID 25993995.
- 1 2 Chen J, Zhang J, Yang J, Xu L, Hu Q, Xu C, et al. (February 2017). "Histone demethylase KDM3a, a novel regulator of vascular smooth muscle cells, controls vascular neointimal hyperplasia in diabetic rats". Atherosclerosis. 257: 152–163. doi:10.1016/j.atherosclerosis.2016.12.007. PMID 28135625.
- ↑ Villeneuve LM, Reddy MA, Natarajan R (July 2011). "Epigenetics: deciphering its role in diabetes and its chronic complications". Clinical and Experimental Pharmacology & Physiology. 38 (7): 451–9. doi:10.1111/j.1440-1681.2011.05497.x. PMC 3123432. PMID 21309809.
- ↑ Harman JL, Jørgensen HF (October 2019). "The role of smooth muscle cells in plaque stability: Therapeutic targeting potential". British Journal of Pharmacology. 176 (19): 3741–3753. doi:10.1111/bph.14779. PMC 6780045. PMID 31254285.
- ↑ "Whole-Genome Chromatin IP Sequencing (ChIP-Seq)" (PDF). Illumina. Retrieved 23 October 2019.
- 1 2 Skene PJ, Henikoff S (January 2017). "An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites". eLife. 6: e21856. doi:10.7554/eLife.21856. PMC 5310842. PMID 28079019.
- 1 2 Meers MP, Bryson T, Henikoff S (16 May 2019). "Improved CUT&RUN chromatin profiling and analysis tools". bioRxiv: 569129. doi:10.1101/569129.
- ↑ Hainer SJ, Fazzio TG (April 2019). "High-Resolution Chromatin Profiling Using CUT&RUN". Current Protocols in Molecular Biology. 126 (1): e85. doi:10.1002/cpmb.85. PMC 6422702. PMID 30688406.
- ↑ Sato Y, Mukai M, Ueda J, Muraki M, Stasevich TJ, Horikoshi N, et al. (14 August 2013). "Genetically encoded system to track histone modification in vivo". Scientific Reports. 3 (1): 2436. Bibcode:2013NatSR...3E2436S. doi:10.1038/srep02436. PMC 3743053. PMID 23942372.